Quality assurance plays a central role in designing and manufacturing biocompatible feedthroughs. SCT Ceramics’ products are used in devices operating in delicate and precious conditions such as the heart, ear or brain. All risks must be eliminated. Our quality processes and inspections leverage equipment and systems which guarantee precision and accuracy without sacrificing speed.
SCT Ceramics’ Medical Division is certified ISO 13485 and our product assembly is conducted in an ISO 8 clean room.
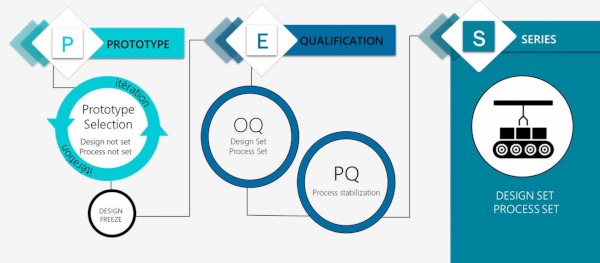
Process Validation
- Our own PES Process (Prototype-Engineering-Series) ensures smooth and efficient transitions across the development timeline so that the end product and manufacturing process is stable, reliable and efficient.
Operational Excellence
- SCT Ceramics’ mission is to guarantee a quality of production and service systematically in line with our customer’s expectations. We strategically use leading continuous improvement methods such as Six Sigma, 5S and SPC to ensure quality at all levels throughout our organization.
Medical Device Regulation
- Our client’s devices are sold around the world. We ensure our feedthroughs and casings allow our clients to meet the strict regulations outlined by the FDA, CE and NMPA (formerly CFDA) as well as respond to ISO 10993 biocompatibility norms.
Product Testing
- We conduct rigorous inspections throughout product development. During mass production, 100% of our feedthroughs are inspected and tested according to the most vital parameters (such as hermeticity) before being shipped. In addition, all batches are sampled at random for additional testing and inspections.
- The non-exhaustive list of inspections include:
- Leak test for hermeticity (100%)
- Electric tests (100%)
- Thermal shock (100%)
- Dimension Inspection (random sampling, AQL standards)

