Sensydia has received the US Food and Drug Administration (FDA) Breakthrough Device Designation for its non-invasive cardiac monitoring device, Cardiac Performance System (CPS).
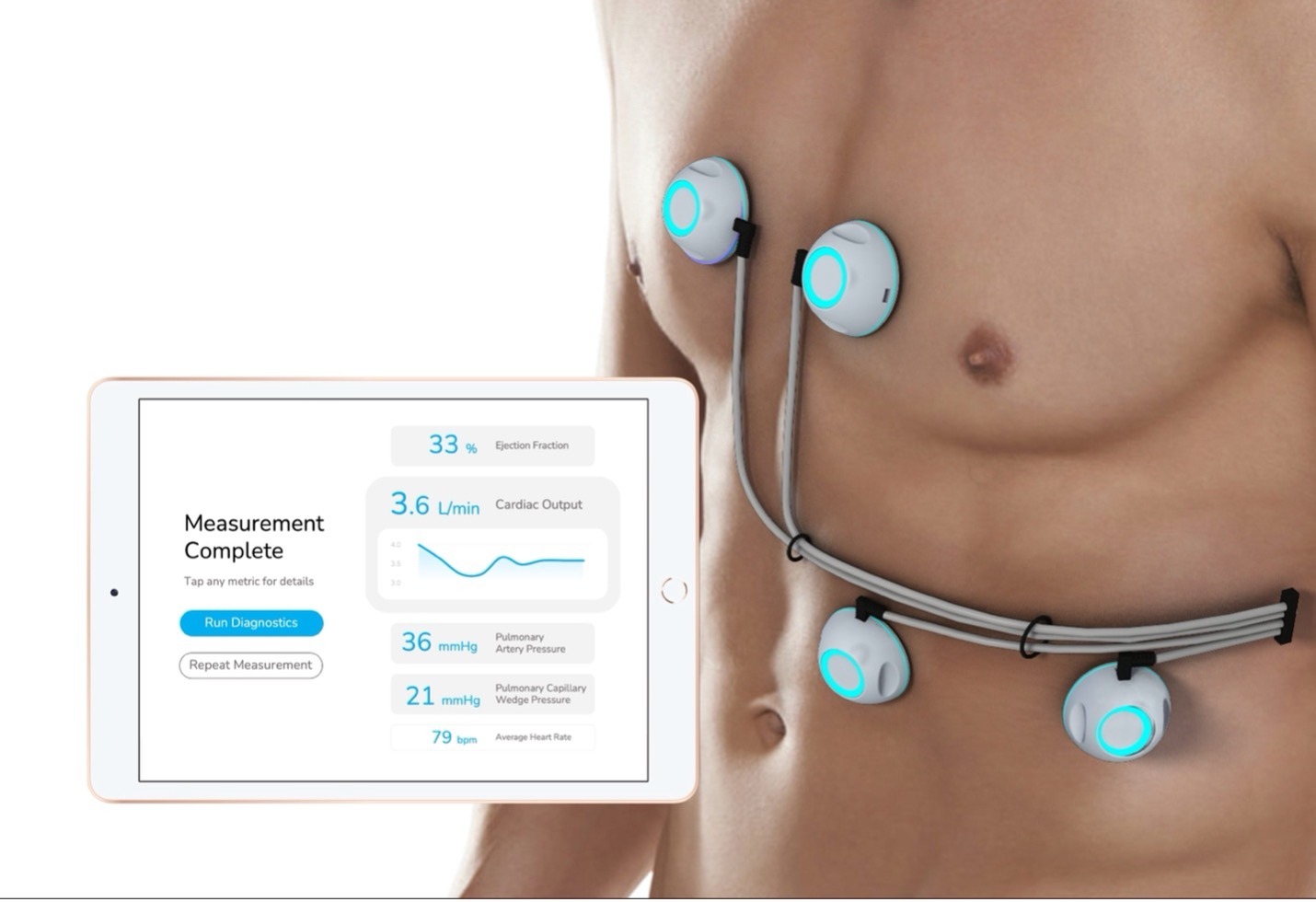
CPS is a non-invasive cardiac monitoring device, designed to report critical heart performance measurements to physicians.
It evaluates patients with advanced and persistent heart failure, eliminating the need for in-hospital catheterisation procedure or ultrasound assessment.
As part of the Breakthrough Device Designation, the US regulator will help advance the development of CPS and prioritise the regulatory review of the device.
Sensydia president and CEO Anthony Arnold said: “Sensydia’s acoustic sensing technology and advanced AI algorithms form the basis of CPS, and together with our stellar research and development capabilities, position it as a breakthrough device.
“This breakthrough designation is a significant milestone for Sensydia. We look forward to working with the FDA to advance the development of CPS and bring it to market in 2023.”
CPS acquires heart sound data through its ultra-sensitive biosensors, and applies machine learning to compute multiple hemodynamic measurements.
It measures ejection fraction, cardiac output, pulmonary artery pressure, and pulmonary capillary wedge pressure, and delivers a comprehensive report via the CPS iPad app.
According to Sensydia, heart failure is a serious public health epidemic, and haemodynamic measurements play crucial role in diagnosis and management of heart disease patients.
Haemodynamic measurements, which define the functioning of a patient’s heart, currently require specialised techniques, and may cause delay in treatment.
Sensydia claimed that its CPS offers accurate and non-invasive hemodynamic assessment within minutes, without needing a highly skilled operator.
The new cardiac monitoring technology enables effective management of patients in the heart failure spectrum without requiring in-hospital procedures or diagnostic tests.
The company has secured the US FDA 510(k) approval for CPS in 2018, for non-invasive measurement of ejection fraction.
Sensydia board member and Colle Capital founder Victoria Grace said: “The approach taken by the Sensydia team towards cardiac care is impressive and second to none.
“We believe this special FDA designation will accelerate our ability to bring this transformational device to market and make a positive impact on the lives of patients.”






