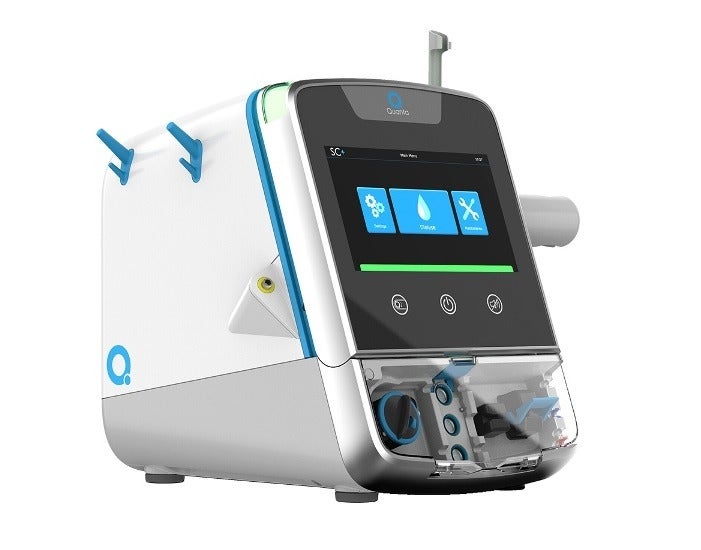
British medical technology firm Quanta Dialysis Technologies has secured 510(k) clearance from the US Food and Drug Administration (FDA) for its haemodialysis system called SC+.
The new system has been designed to offer a dialysis dose equivalent to the current standard of care, as well as in a compact and easy-to-use format suitable for a range of care settings.
Quanta’s system has the potential to deliver improved dialysate flow rates compared to other portable haemodialysis systems that offer conventional three-times-a-week prescriptions, according to the company.
The small and lightweight design of SC+ makes it portable and provide efficient dialysis treatments to the patients, while the simple user interface enables a range of healthcare professionals to access dialysis.
Quanta to launch related products to complement SC+
Quanta is also planning to launch a suite of related products and services to complement SC+ haemodialysis system.
The company will introduce an optional and portable water purification module to help SC+ easily move around and operate in a range of settings with and without a centralised water system.
Quanta will also offer a secure and cloud-based digital health offering, which helps to simplify and automate treatment data capture and reporting, thereby helping to avoid the need for creating and storing manual records.
SC+ was commercially launched in the UK last year following broad clinical piloting with the National Health Service (NHS) in England.
Quanta CEO John Milad said: “Kidney failure is one of the costliest health conditions for the American health system. The imperative for better value in healthcare, coupled with new challenges driven by Covid-19, has amplified the need to reimagine how hemodialysis is delivered across a variety of settings.
“This will be a pivotal year for Quanta as we build our US presence and bring our next generation hemodialysis system to a community ripe with need.”
In July 2019, Quanta secured £38m ($48m) in a first closing of its Series C funding round. The financing round was participated by existing investors was led by a Swiss private family office and btov Partners. It was co-led by Wellington Partners and Seroba Life Sciences.






