Prellis Biologics today announced that Khosla Ventures has led an $8.7 Series A investment in the company. The announcement comes as Prellis has reached major tissue engineering milestones in its mission to use 3D holographic printing to create 3D tissue and organs for research and transplantation.
Prellis Biologics’ original seed round investors, including True Ventures and Indie Bio (SOSV), joined Khosla in the round. Invested capital in Prellis now totals $10.5 million.
“Regenerative medicine has made enormous leaps in recent decades. However, to create complete organs, we need to build higher order structures like the vascular system,” said Dr. Alex Morgan, Principal at Khosla Ventures. “Prellis’ optical technology provides the scaffolding necessary to engineer these larger masses of tissues. With our investment in Prellis, we’re supporting an initiative that will ultimately produce a functioning lobe of the lung, or even a kidney, to be used in addressing an enormous unmet global need.”
“The holy grail of human tissue engineering is the ability to build complex tissues with working vascular systems,” said Dr. Melanie Matheu, Prellis Biologics’ co-founder and CEO. “The future of regenerative medicine revolves around harnessing the power of our own cells as therapeutics and building the tissues to keep them alive. Khosla Ventures is the perfect investor to support our merging of deep tech and cutting-edge regenerative medicine. With this technology in hand, we can begin to ask questions about real 3D cell biology that have never been asked before.”
Prellis Reports Progress in Tissue Engineering
The new funding coincides with Prellis’ recent advancements in tissue engineering.
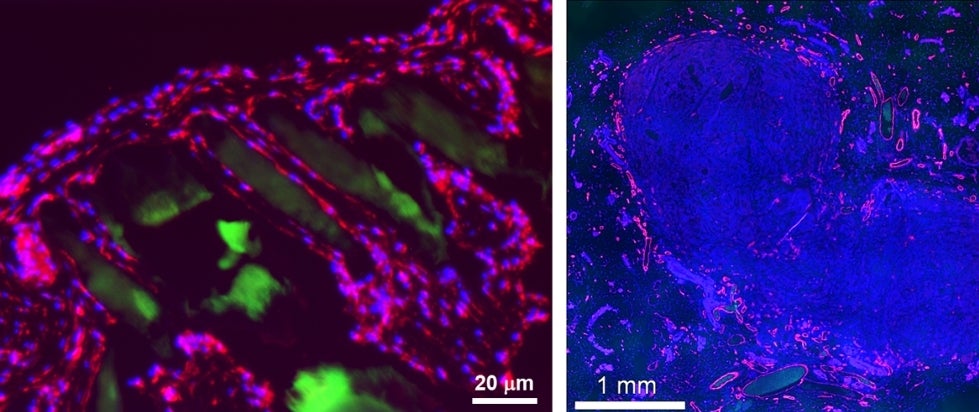
First, Prellis Biologics has demonstrated positive results from the first animal transplantation of its 3D tissue scaffolds – called Vascular Tissue Blanks – carried out at Stanford University.
Prellis’ transplanted structures support human tumor growth at rates that are similar or better than typical tumor transplant methods, but with fewer cells. “We were excited to see that we could achieve full tumor engraftment and vascularization by transplanting just 200,000 cells. This is an order of magnitude fewer cells, since typical tumor studies in animals require two million or more cells,” said Dr. Matheu. “A breakthrough like this opens the door to studying rare human tumors and complex human tumor immune system reactions. It has the potential to significantly reduce overall animal use and speed up drug discovery efforts.”
A critical finding in the animal studies was confirmation that the laser-printed structures support rapid and extensive vascularization. Within two weeks, Prellis-built scaffolds were populated by 10-micron capillaries that grew spontaneously, even without the presence of pre-seeded cells. Within eight weeks, large, branched vasculature up to 50 microns was identified deep inside of the transplanted structures, indicating the animal’s vasculature system had adopted the scaffolding and incorporated it into its own circulatory system.
“Spontaneous, structurally guided vascularization of our laser-printed structures is a significant milestone on the way to transplanting complex tissues. We’ve demonstrated that we can establish circulation in our transplanted structures,” said Dr. Matheu. “Our team will be compiling the results of these studies for peer review publication later this year.”
Second, Prellis is the first company to deliver biocompatible vascularized tissue blanks to the pharmaceutical and academic markets for advanced 3D tissue culture. Prellis’ Vascular Tissue Blanks are now being used by scientists for research in oncology, tissue development research, neurobiology studies, and drug testing. Over 30 pharmaceutical and academic research labs, including groups at UC San Francisco, Johns Hopkins, UC Irvine, and Memorial Sloan Kettering, are experimenting with the Vascular Tissue Blanks, which allow therapeutics to be tested in consistent and fast-to-set-up models of 3D human tissues for the first time.
“We had been using grow-your-own spheroid techniques, which required intricate setups and were inconsistent in size and morphology, complicating downstream analysis,” said Maria Soloveychik, PhD, CEO of SyntheX. “What changes with Prellis? You just use it. The setup is quick and consistent, and the handling is straightforward.”
Prellis’ Vascular Tissue Blanks reduce time to drug screening in 3D organoids by an estimated 90% relative to other 3D cell culture technologies. The organoids are fully transplantable just a few hours after the cells are added. With the pre-made tissue scaffolds, 3D organoid drug screening results can be achieved in as little as 48 hours. Prellis Biologics is the only company capable of producing high-resolution (< 1 micron) 3D printed structures out of biocompatible hydrogels.
“The tissue blanks are designed to promote oxygenation of high-density cell cultures grown in 3D. We use them in our own laboratory to study organ development. It’s exciting to provide them as a ready-made solution for the 3D cell culture drug screening process. We haven’t found a human cell type that won’t grow on our 3D scaffolds,” said Dr. Matheu.
Third, Prellis has made significant progress in achieving in vitro microfluidic flow through complex vascular channels, with separation as fine as 10–20 microns – a critical precursor to creating complex viable vascularized tissue in the laboratory. Prellis’ R&D team is currently testing 10-20 micron biomaterial interface for cell-cell interactions and nutrient exchange – the first steps in ensuring a functional filtration and gas exchange system, critical building blocks for organ systems such as the lung and kidney.
Ultimately, Prellis Biologics has its sights set firmly on human organ transplantation and expects to initiate its first large animal studies before the end of the year. The first transplanted large tissues Prellis will test are engineered arterial replacements, 3-4 mm in diameter. “Arterial replacements are a natural stepping-stone to production of larger solid organs,” said Dr. Matheu. “The ones we have designed are far more sophisticated than the standard vascular replacements. They are surrounded by fine capillary beds that are known to contribute to the structural integrity and engraftment of arteries after the surgical procedure.”
Source: Company Press Release






