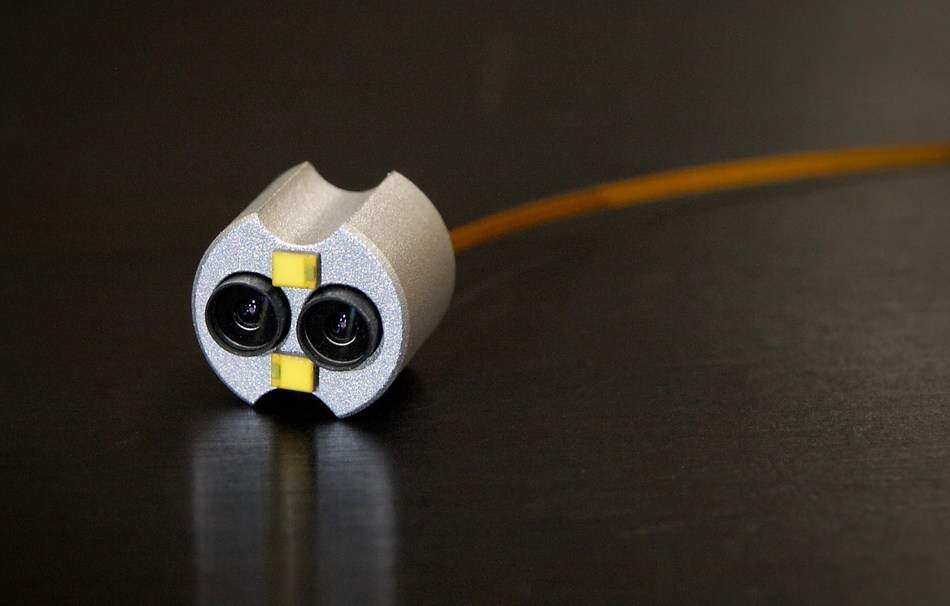
The platform, which allows medical device manufacturers to integrate stereoscopic 3D imaging into endoscopes, incorporates CMOS image sensors, image signal processor (ISP), a light control board and a camera control unit.
OmniVision provides two of the CMOS image sensors and an ISP, while Lighthouse offers light control board and a camera control unit required for the platform.
OmniVision principal marketing manager Tehzeeb Gunja said: “Traditional endoscopy uses a single camera and does not provide depth information, thus increasing risks during endoscopic surgery. This partnership with Lighthouse Imaging provides an end-to-end, turnkey solution for stereoscopic 3D imaging in applications such as laparoscopy, gastro-intestinal (GI) and general endoscopic surgery.
“OmniVision’s high-image-quality, high-resolution image sensors, combined with Lighthouse’s system-level integration expertise, allows us to overcome the complexity, cost and size constraints that have limited the wide adoption of 3D endoscopy.”
Two OmniVision OH02A image sensors with 1.4-micron pixel PureCel technology in a 1/6-inch optical format are used in the platform to support alternate-row high dynamic range for high-quality imaging of both bright and dark areas.
OmniVision said that the image sensors in the platform are designed to be stereo-ready, with FrameSync technology and provides precise synchronization of the sensors.
The 2mp, 1080p resolution of the OH02A image sensor supports streaming video at 60fps and facilitates the entire medical team to view high-quality images on a large television monitor. The Lighthouse platform is capable of adapting easily with OmniVision sensors with 720p or 4K/2K resolution.
The OVMed ISP back-end processor is designed to be compact and fits inside the camera control unit/video processor unit to provide stereo 3D vision. It supports an integrated design including analog-to-digital converter, and dual-channel ISP and PC interface.
Lighthouse Imaging CEO Robert Austring said: “At Lighthouse Imaging, we are passionate about developing and manufacturing custom optical and digital imaging solutions for medical device companies. With this partnership, leveraging OmniVision’s OH02A sensor and imaging expertise, we are now able to meet and exceed the high demands of our clients worldwide.
“OmniVision’s technologies allow us to immediately integrate quality image sensors into our systems and scale to create the next generation of products.”
The medical devices designers can integrate the platform directly into a design to reduce costs and leads to an easy 510(k) clearance. This system is also enabled with image sensors, lenses or illumination customizable switching.
The platform manufacturers claim that OmniVision’s OH02A sensor is in line with RoHS and REACH, and passes autoclave testing. The Lighthouse demonstration units are compliant with IEC and FDA regulations, and are ISO-13485 certified to reduce development risk.






