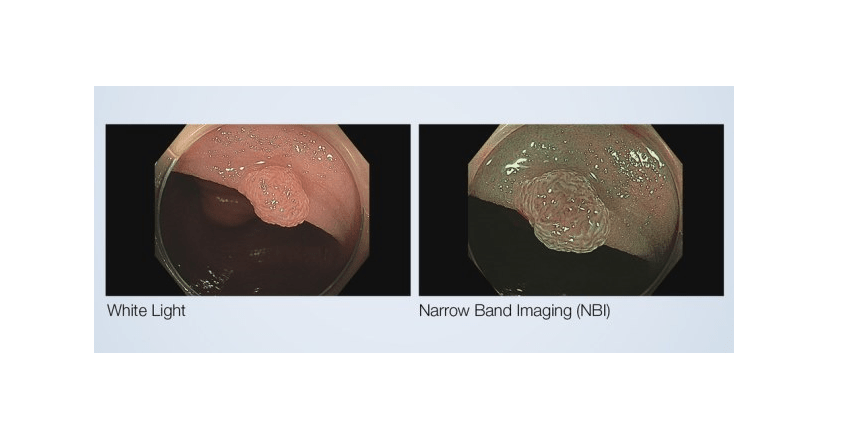
Olympus has secured 510(k) clearance from the US Food and Drug Administration (FDA) for its Narrow Band Imaging (NBI) to evaluate the neoplastic potential of colorectal polyps.
The company said that physicians are enabled to better predict histology for polyps 5mm or smaller known as diminutive polyps, with the application of NBI International Colorectal Endoscopic (NICE) classification during a screening colonoscopy.
The FDA’s approval is based on a meta-analysis of prospective real-time clinical studies of NBI use during colonoscopy.
According to the company, the data showed that NBI demonstrated 93% sensitivity and 85% specificity in predicting adenomatous histology of diminutive polyps during colonoscopy as well sd over 90% negative predictive value in assigning post-polypectomy patient surveillance intervals following colonoscopy.
NBI is an optical imaging technology, which improves the visibility and contrast of vessels and surface patterns on the mucosa.
The technology filters out all but blue and green light which are absorbed by hemoglobin and penetrate only the surface of human tissue, while white light uses all colours in the spectrum.
NBI is also suitable for clinical applications in urology, pulmonology and rhinolaryngology (ENT),
Olympus already secured FDA clearance for NBI for the screening and surveillance of Barrett’s esophagus, in addition to the current indications.
The company is also currently exploring other claims for NBI both in gynecology and general surgery.
Olympus America endoscopy vice president Kevin Mancini said: “We are excited about these newly cleared indications for using NBI to support colorectal cancer screening.
“NBI is an important tool, available as standard on all Olympus colonoscopes, which can be used by physicians to assist in decision making with the goal of improving patient care.”
In February, the company partnered with the SeleCT QCT analysis service to evaluate patient eligibility for treatment with the Spiration Valve System.
Spiration is an FDA-designated breakthrough device to treat severe emphysema, a form of chronic obstructive pulmonary disease (COPD).






