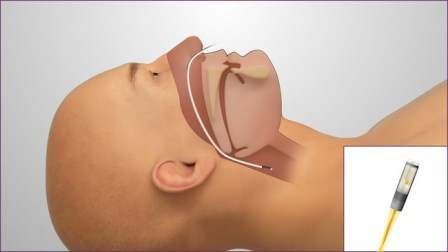
The CE mark for Mikro-Cath has been expanded to include airway and intra-compartmental pressure measurements, which will allow European physicians and clinical researchers to access high-fidelity pressure data.
Earlier, the Mikro-Cath secured approval for cardiovascular pressure measurements in Europe. In March 2017, the company also secured approval from the US Food and Drug Administration (FDA) for the expanded indications.
Mikro-Cath was originally designed as a cardiovascular medical device, and the new expansion will enable European physicians to make improvements in respiratory care and provide continuous monitoring technique to measure compartment pressure and accurately diagnose acute compartment syndrome.
The device can also be incorporated into various critical care programs involving trauma patients undergoing ventilation treatment.
The enhanced level of continuous data accuracy for both respiratory and compartment pressure applications will enable quick decision making with improved data.
Millar international sales and business development director Matthew Davis said: “This has been a long awaited approval and we look forward to working with physicians that have been eager to utilize the Mikro-Cath for these applications.
“We anticipate that this will open an opportunity for an improvement in patient care, specifically in the respiratory market.”
The company secured ISO 13485:2016certification for its quality management system, after completion of an audit by British Standards Institute (BSI).
BSI is an international governing body for quality medical device manufacturing.
Millar has produced MEMS sensor-enabled catheters that are used to measure physiologic pressures, and were used to optimize advance medical research and device development in cardiovascular, urodynamic, neurosurgical care and orthopedic applications.
Since 1969, the company has been involved in the development of catheter-based and solid-state pressure sensors. Its precision catheter-based technology will help measure high-fidelity physiological parameters to improve accuracy in patient evaluation.
In addition, the firm offers OEM solutions to the customers in the medical device and life sciences industries.






