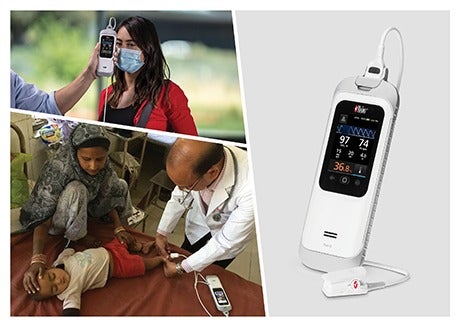
Medical technology company Masimo has secured CE mark approval for a rugged handheld device called Rad-G with Temperature.
The Rad-G with Temperature offers SET pulse oximetry, respiration rate from the pleth (RRp) and other major parameters alongside clinical-grade, non-contact infrared thermometry.
With its integrated noninvasive and real-time forehead temperature measurement capabilities, the handheld device allows clinicians to rapidly evaluate patients and make informed care decisions.
The Rad-G with Temperature, which is incorporated with the Mini-Clip pulse oximeter sensor, is designed for use in different settings such as entry screening, physicians’ offices, outpatient services, long-term care facilities, and wellness clinics.
In addition, the device is also suitable for use in first-response scenarios and limited-resource environments both indoors and in the field.
According to Masimo, the handheld device has the potential to deliver both spot-check measurement and continuous monitoring.
The company stated that the Rad-G was first developed in collaboration with The Bill & Melinda Gates Foundation as a spot-check device for use in pneumonia screening.
Rad-G enables its conversion from a handheld and spot-check device into a continuous monitoring device with a power adapter, when other multi-parameter monitors are not available.
Masimo founder and CEO Joe Kiani said: “With Rad-G, we set out to create an accessible, high-quality care solution that clinicians can rely on in a multitude of care settings to serve the five billion people on our planet that to date have not had access to pulse oximetry, let alone SET pulse oximetry.
“With the addition of temperature measurement, Rad-G is more versatile than ever, streamlining the assessment of multiple key vital signs.”
The SpO2 and PR monitoring on Rad-G is offered with the support of Masimo SET Measure-through Motion and Low Perfusion pulse oximetry.
In addition, the Rad-G with Temperature is suitable for use with a variety of reusable and single-patient use sensors. It is compatible with different Masimo single-patient-use adhesive sensors such as Masimo RD SET sensors.
Masimo already secured 510(k) clearance from the US Food and Drug Administration (FDA) for the Rad-G device. The Rad-G with Temperature has not yet approved by the FDA.
In October last year, Masimo introduced a new wearable and wireless Radius T° continuous thermometer, which continuously measures data and transmits data and customisable temperature notifications to the user’s smartphone.






