US-based medical technology company Masimo has received the US Food and Drug Administration (FDA) 510(k) approval for its Rad-G with Temperature device.
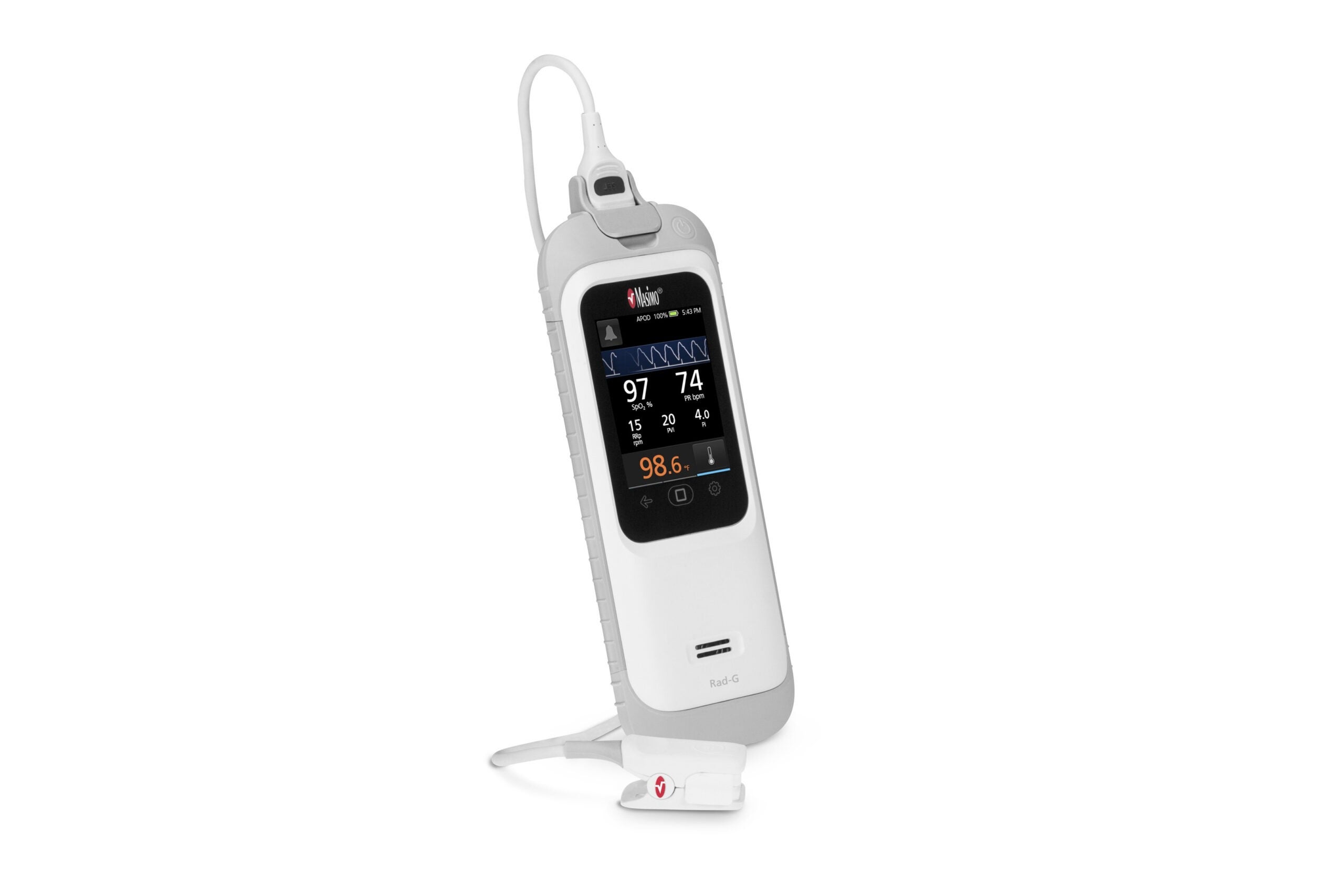
The Rad-G with Temperature is a rugged, handheld monitor that provides SET pulse oximetry, respiration rate from the pleth (RRp) and other key parameters.
It is a light-weight device that comes with a long-lasting rechargeable battery, sturdy rubber casing, along with integrated non-invasive infrared clinical thermometry.
Rad-G with Temperature can be used in various settings, including physicians’ offices, outpatient services, urgent care facilities, wellness clinics, and in first-responder scenarios.
It enables care teams to rapidly measure vital signs using a single, compact, portable device and make informed decisions anywhere, said the medical technology company.
Masimo founder and CEO Joe Kiani said: “With Rad-G, we set out to create an accessible, high-quality care solution that could be used in a multitude of care settings to serve the five billion people on our planet that to date have not had access to pulse oximetry, let alone SET pulse oximetry.
“With the addition of temperature measurements, Rad-G is more versatile than ever, streamlining the assessment of multiple key vital signs.
“With this FDA clearance, Rad-G with Temperature can now be deployed across the US, in addition to many other parts of the world, helping support clinicians in almost any care scenario.”
Rad-G with Temperature offers a non-contact infrared thermometry technology, which does not require probe covers or other disposable accessories.
Infrared thermometry facilitates easy and effective temperature screening with one-touch operation, alongside oxygen saturation, respiration rate, and more, using a single device.
Its integration into the Rad-G platform eliminates the need for clinicians to use a separate clinical thermometer for measuring body temperature.
Rad-G’s rechargeable battery provides 24 hours of operational use between charges, allowing clinicians to work in transit, emergency, and other challenging scenarios.
Masimo developed Rad-G with Temperature in partnership with The Bill & Melinda Gates Foundation, as a spot-check device for use in pneumonia screening, and launched it outside the US.
The device is compatible with the broad portfolio of its single-patient-use adhesive sensors, including Masimo RD SET sensors, said the company.






