The CE mark certifies a medical device’s conformity with health, safety, and environmental protection directives, enabling them to be sold within the European Economic Area (EEA).
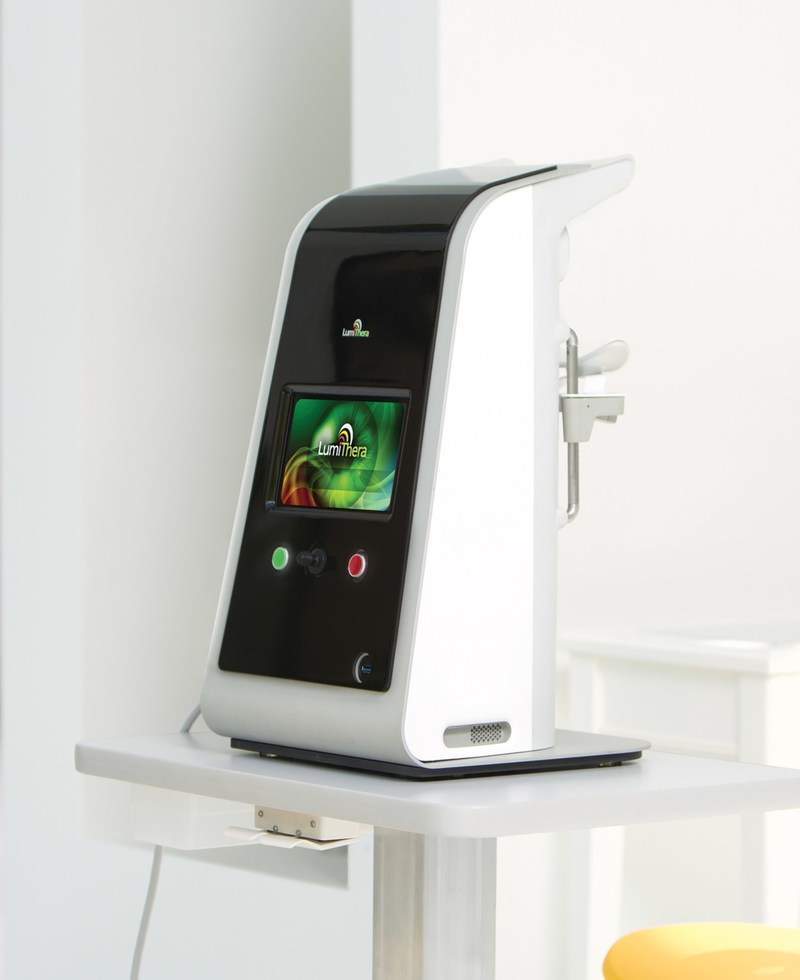
The LT-300 Light Delivery System is a non-invasive photobiomodulation (PBM) therapy being developed by the US-based clinical stage medical device company for the treatment of sight threatening, acute and chronic ocular disease.
LumiThera president and CEO Clark Tedford said: “The CE mark allows LumiThera to begin commercialization throughout the 28 EU member states and coincides with the initiation of the LIGHTSITE II Clinical Study in select European sites in the upcoming months.
“We are excited to be able to offer a safe and effective early stage clinical intervention for patients with dry AMD.”
Last month, LumiThera reported positive results for the LT-300 Light Delivery System from a clinical trial LIGHTSITE I in patients with dry AMD condition.
The trial was a 30-subject prospective, randomized, double masked, pilot study that assessed each patient’s vision and studied disease pathology in the eye, after giving them PBM treatments for up to 12 months.
The PBM treatment using the LT-300 device succeeded in considerably bringing down the central drusen volume through the one-year study in comparison to sham, following one year of treatment. Drusen is the major pathology of dry AMD and is thought to be a crucial proinflammatory mediator and marker for disease progression.
University of Toronto UHN retinal services ophthalmologist-in-chief and director Robert Devenyi said: “Until now we just waited and watched the patients lose vision with limited therapeutic options. Now we may have a treatment that can target improvement in visual outcomes and reduce a key component of the pathology.
“The next step is to confirm the early results in the multi-center LIGHTSITE II trial and work with ophthalmology medical community in establishing best treatment practices.”






