LifeSignals has announced plans to fast track the launch of a single use, wireless medical biosensor patch for COVID-19 (coronavirus) patient monitoring.
The new-to-market biosensor technology is claimed to be the first of its kind to overcome the restrictions of using wireless devices within a hospital environment.
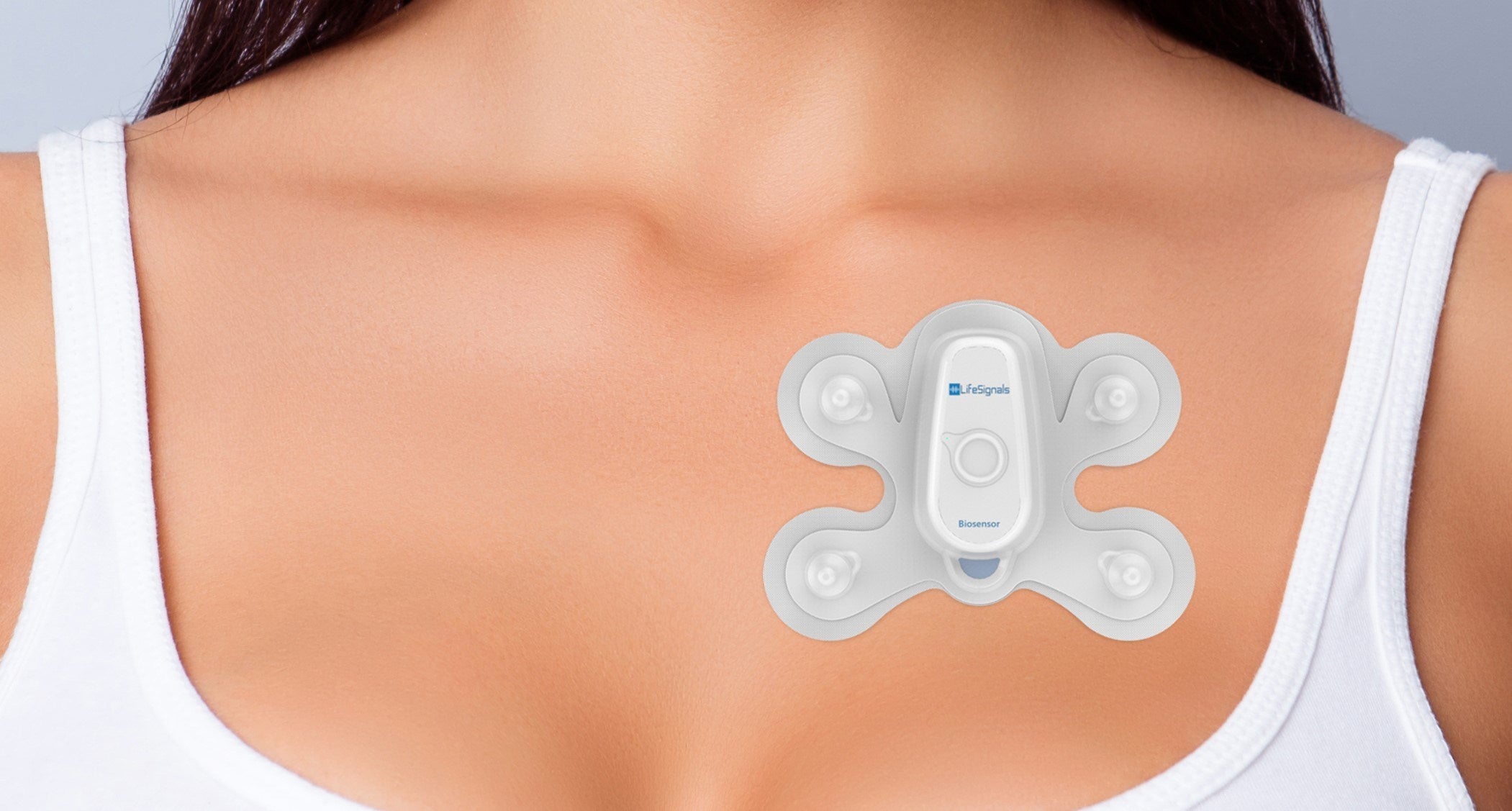
Self-affixed on the chest for five days, the Biosensor Patch 1AX, which is based on a proven cardiovascular monitoring platform, will record temperature, respiration rate, ECG trace, heart rate and movement in real time.
According to LifeSignals, data will be sent wirelessly from the patch and displayed in real time on an app on the user’s phone.
If symptoms develop, the device and its data platform can alert healthcare workers to take additional action.
The company added that the data can be sent to a secure cloud platform allowing healthcare authorities to initiate successful mass population remote screening for groups including those in quarantine, patients in care home facilities and vulnerable high risk people in their own households.
LifeSignals is also planning to launch the Biosensor Patch 2A in June
LifeSignals also plans to roll out the Biosensor Patch 2A in June, which captures, stores and streams clinical-grade vital signs data including SpO2.
This updated patch will enable monitoring COVID-19 patients recovering in intensive care units and clearing them to be moved to other wards or off-site.
It will also allow the patient’s vital sign data to be continuously monitored in real-time at their homes.
In the process, this patients will also help in optimising use of scarce medical resources and free up hospital beds.
LifeSignals co-founder and CEO Surendar Magar said: “We identified two key areas where healthcare systems are choked – consumers calling in about symptoms they are experiencing and lack of critical care hospital beds – and have designed these two biosensor patches which are suited for mass production.”
“To ensure the new biosensor patches become widely available, LifeSignals have created a fully interoperable technology that can easily be integrated into most health monitoring platforms, apps and healthcare systems.
“We are actively seeking companies and organisations to partner with to bring clinical-grade remote monitoring to a much wider patient base, helping reduce the burden on healthcare systems.”
In July 2018, LifeSignals had received FDA clearance for its wireless LP1100 Life Signal Patch for enabling the next generation of wearable, healthcare monitoring devices.






