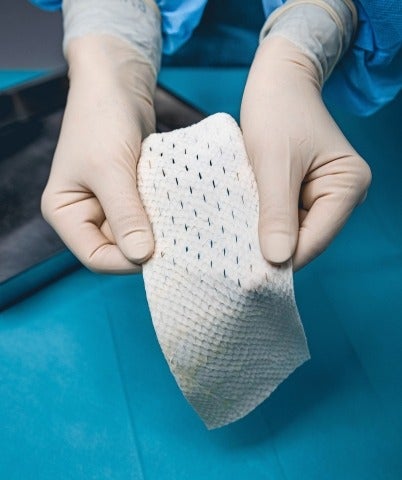
Kerecis, a provider of Omega3-rich fish skin-based products, has received the US Food and Drug Administration (FDA) marketing authorisation for its Omega3 SurgiBind.
Omega3 SurgiBind is an implantable fish-skin graft intended for use in plastic and reconstructive surgery, and is currently available in the US.
Its implantation is indicated for strengthening weak soft tissue in patients who require soft tissue repair, or reinforcement in plastic or reconstructive surgery.
SurgiBind product was first commercially used outside of a study, at Atrium Health’s Sanger Heart and Vascular Institute, by surgeon Dr. Hector Crespo Soto.
Soto said: “I used the product in a ray amputation surgery, and I was pleased with the outcome. There were no post-op complications; the incision stayed closed and healed quickly.
“The Kerecis product compliments surgical procedures, provides a bacterial barrier and reinforces the body’s healing mechanism.”
Omega3 SurgiBind is said to help medical practitioners to better manage the risk of complications and improve outcomes.
The fish-skin technology helps in rapid incorporation, cell ingrowth, speeding up neovascularisation and wound closure, which are expected to boost full tissue remodelling, said the company.
It finds potential applications in strengthening of soft tissues in abdominal incisions, surgical flaps and hip arthroplasty.
Kerecis Omega3 is an intact fish skin that uses the body’s own cells, when grafted onto damaged human tissue, to convert it into living tissue.
According to the company, the Kerecis fish skin retains its similarity to human tissue, as no disease-transfer risk exists between cold-water fish and humans.
Kerecis is engaged in developing products based on fish skin and fatty acids for cellular therapy, tissue regeneration and protection.
Alongside SurgiBind, the company also offers three other products for surgical applications, including Kerecis Omega3 SurgiClose, SurgiClose Micro and GraftGuide.
Kerecis founder and CEO Fertram Sigurjonsson said: “This new product re-enforces our entry into the surgical market and demonstrates that the benefits of our technology extend beyond treating severe wounds and preventing amputations.”






