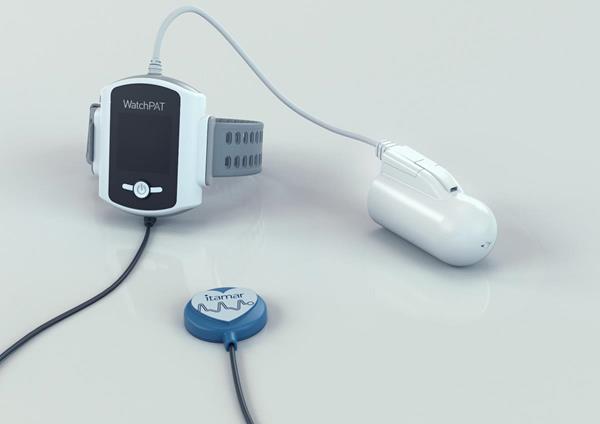
WatchPAT 300 serves as a cost-effective method for rapid, scalable and effective diagnosis of sleep apnea, enabling physicians to better treat its patients.
By using peripheral arterial tone and other signals, WatchPAT 300 will calculate true sleep time and complete sleep architecture to precisely and reliably diagnose both obstructive and central sleep apnea.
The design of WatchPAT 300 includes three points of contact and without cumbersome nasal canula or chest belts to improve ease of use for the patients.
Based on the peripheral arterial tone signal (PAT), the next generation WatchPAT system uses an advanced actigraphy to differentiate between wake and sleep periods to calculate true sleep time.
The PAT amplitude and pulse rate will also be used by the WatchPAT 300 to differentiate between non-rapid eye movement (REM) and REM sleep.
The system is also said to offer clinically validated sleep architecture, based on sleep stages, including sleep efficiency, sleep latency and REM latency.
WatchPAT 300 also features Central+ module that facilitates specific identification for central sleep apnea events.
In August 2018, the company secured 510(k) clearance from the US Food and Drug Administration. It has been developed to gradually replace the current WatchPAT 200 platform.
WatchPAT 300 is also compatible with zzzPAT software and CloudPAT cloud-based IT solution for convenient sleep diagnosis and secure patient data transfers to streamline workflow.
The company will exhibit the WatchPAT 300 system at SLEEP 2019 event in San Antonio of Texas, which will take place between 8 and 12 June.
Itamar Medical president and CEO Gilad Glick said: “Addressing the challenge of efficiently and cost-effectively diagnosing the millions of patients with undiagnosed sleep apnea requires a simple, accurate and scalable modality.
“We believe that WatchPAT 300 – which demonstrates Itamar Medical’s continued commitment to innovation focused on meeting the needs of patients, health systems, payers and physicians – can play a critical role in solving this challenge.”






