US-based nanosensor technology firm IdentifySensors Biologics has developed a fast, accurate, over-the-counter RNA COVID-19 self-test technology for Smartphone.
The new technology detects COVID-19 RNA in saliva through newly developed electronic nanosensors, which reduces error rates that are associated with chemical-based home tests.
The company said that the over-the-counter self-test that can be performed at home, is now in its final stages of development at Purdue University.
The electronically based test, Check4-COVID will not require any prescription, nasal swabs, mailed samples, or waiting in line.
Check4-COVID technology detects asymptomatic and early infections on a molecular level
This has the ability to accurately detect asymptomatic and early infections on a molecular level, which is a significant weakness in home tests.
IdentifySensors CEO Gregory Hummer MD said: “Chemical-based home tests do not detect the virus on a genetic level and often miss early and asymptomatic cases.
“By the time someone gets sick and tested, the virus usually has already spread. Our test catches early infections and asymptomatic cases within minutes and without clinical intervention. This is a major breakthrough in managing the pandemic.”
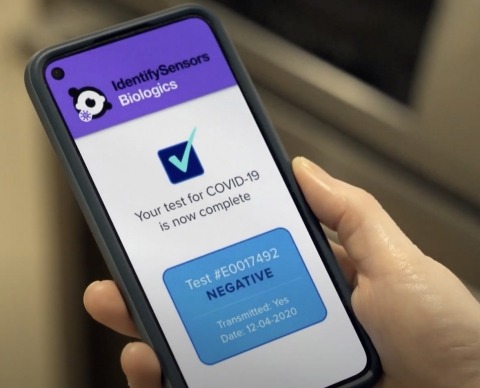
The company said that the test sends the accurate RNA results to a user’s smartphone within minutes.
Upon receipt of FDA approval, which is expected early next year, the new test is expected to be an over-the-counter, entirely at-home self-test that meets or exceeds the gold-standard in lab-grade testing.
Purdue Institute of Inflammation, Immunology and Infectious Disease director Richard Kuhn said: “This new platform technology takes pathogen testing down a completely different path than all the other diagnostic tests out there now. Our COVID-19 testing research is showing very promising results.”
According to IdentifySensors, each COVID test cartridge is estimated to cost less than $25 and allows the consumers to test themselves instantly at home without waiting in line.
It is also developing additional test cartridges for other pathogens including influenza A and B that can be used in the same reader.






