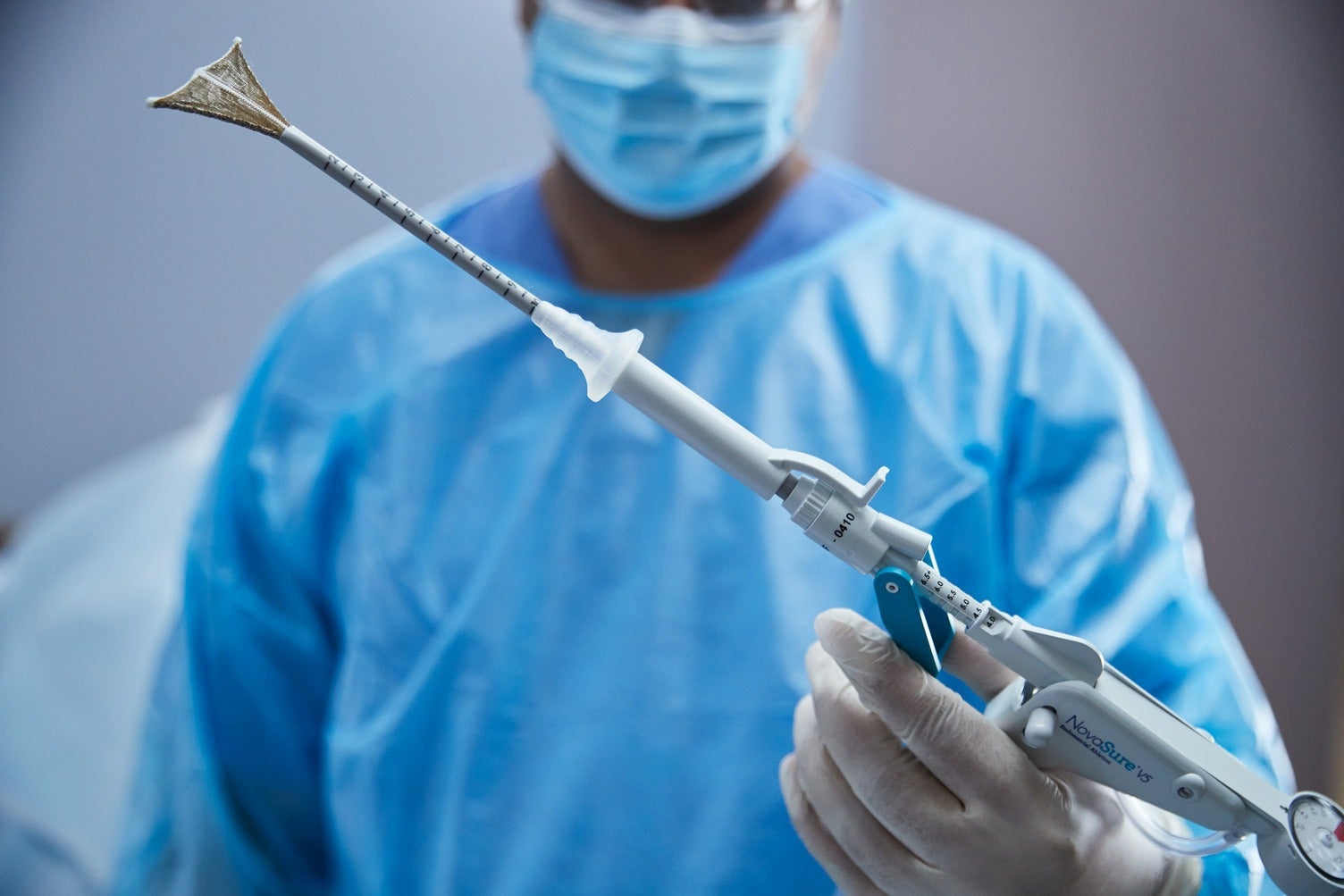
Hologic, a medical technology company focused on women’s health, has secured regulatory approval for its NovaSure V5 global endometrial ablation (GEA) device in Canada and Europe.
The advanced, new-generation device incorporates enhanced features designed to treat a wide range of cervical and uterine anatomies.
It features the company’s patented NovaSure technology to reduce or stop menstrual bleeding for women with Abnormal Uterine Bleeding (AUB).
NovaSureV5 GEA device comes with a patient-centric design, and can treat a wide variety of patients more effectively, the company said.
Hologic group international president Jan Verstreken said: “Since its launch over 20 years ago, we have constantly listened to our customers’ feedback, which has driven continuous design improvements for NovaSure.
“This has enabled us to develop our technology to further meet their needs and those of the women they treat, ultimately delivering better outcomes for these women.”
NovaSure V5 features EndoForm technology, which increases the sealing surface and accommodates a range of cervical canals and anatomical variability.
The device comes with AccuSheath markings that are designed to enhance the accuracy and confidence of seating and fundal placement.
In addition, NovaSure V5 is equipped with SureClear technology, the company’s unique fluid removal system, and SmartDepth technology.
SureClear technology provides integrated suction through constant tissue contact while simultaneously removing ablation by-products such as vapour and fluid.
The device enables an endometrial ablation procedure for AUB treatment with 97% patient satisfaction and 87% of patients avoiding a hysterectomy at 10 years, said Hologic.
CHU de Québec-Université Laval in Québec professor Philippe Y Laberge said: “I welcome the new features on the NovaSure V5. These will support both inexperienced and more established users in performing effective, more efficient and accurate procedures.
“The addition of markings to the shaft will enable users to correctly place the device before initiating a procedure.
“The cervical seal is a significant improvement as there is less chance of leaks, allowing the completion of pre-procedure checks and the procedure itself.”






