Medical technology company Hologic has secured CE mark approval for its new Genius digital diagnostics system for cervical cancer screening.
Genius digital diagnostics is a digital cytology platform that helps cytotechnologists and pathologists detect pre-cancerous lesions and cancer cells in women, by integrating a new artificial intelligence (AI) algorithm with advanced digital imaging.
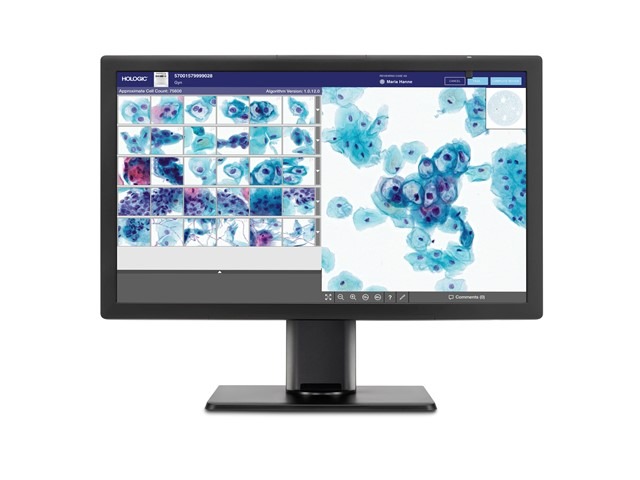
The system includes a digital imager for image acquisition, an AI algorithm for analysing images, an image management server for storing images and a review station for case review.
Genius system offers gallery view of most diagnostically relevant images
The new system has the capacity to analyse all cells on a ThinPrep Pap test slide, thereby facilitating a gallery view of the most diagnostically relevant images.
According to the company, the system offers healthcare providers with the crucial information they require to guide earlier detection and better treatment decisions for the patients.
The Genius digital diagnostics system offers digital case review to help lab partners enhance workflow and speed up review time.
Hologic already offers liquid-based cytology technology called ThinPrep Pap test and FDA-approved mRNA-based HPV test called Aptima HPV assay for cervical cancer screening.
Hologic diagnostic solutions division president Kevin Thornal said: “Application of AI requires digital images that are of exceptional quality.
“Our teams developed a breakthrough imaging technology that converts physical glass cytology slides into digital images with superior clarity. From this digitalisation, advanced image analysis and improved standardization are now achievable.”
In August, Hologic agreed to acquire women’s health company Acessa Health for around $80m in cash.






