Medical technology company Hologic has unveiled plans to introduce a new Aptima molecular assay for the detection of novel coronavirus (SARS-CoV-2).
Designed to run on the company’s Panther system, the research use only (RUO) version of the Aptima SARS-CoV-2 test will be distributed to the hospital, public health and reference laboratories certified under the Clinical Laboratory Improvement Amendments (CLIA) act.
The CLIA-certified labs will use the assay for clinical testing on Hologic’s Panther system
Upon completion of the performance verification testing, the CLIA-certified labs will use the assay for clinical testing on Hologic’s Panther system.
The company is also planning to file for emergency use authorisation (EUA) for the Aptima SARS-CoV-2 assay with the US Food and Drug Administration (FDA). In May, the company is planning to apply for a CE mark for diagnostic use in Europe.
Initially, the company is planning to supply around three million RUO tests for laboratory customers.
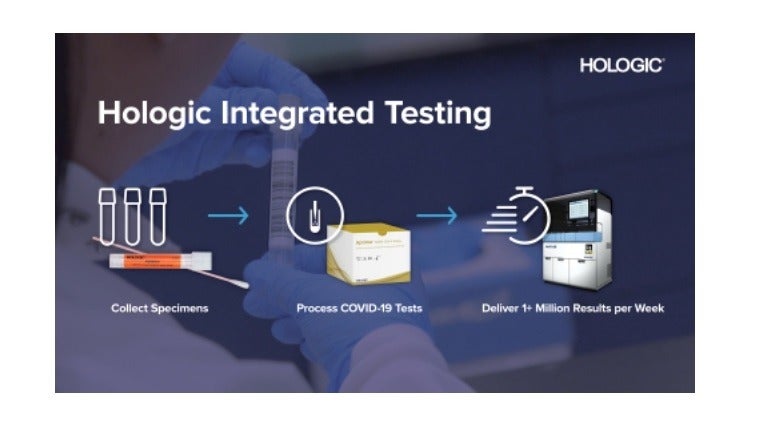
From late May, Hologic intends to commence production of around one million Aptima SARS-CoV-2 assays per week on average. It also aims to increase its production capacity further in the coming months.
Hologic’s Panther system is a fully automated and high-throughput molecular diagnostic platform, which can offer initial results in around three hours and process over 1,000 coronavirus tests within one day.
According to the company, around 750 US hospitals, public health and reference labs deployed the Panther system and its suite of Aptima technologies such as transcription-mediated amplification (TMA) to conduct molecular tests for sexually transmitted infections, cervical cancer screening and viral load monitoring in people with HIV and hepatitis.
Hologic chairman, president and CEO Steve MacMillan said: “Our second COVID-19 test leverages the same proprietary Aptima chemistry and Panther instrumentation that have made Hologic a leader in molecular diagnostics for other infectious diseases.
“The ability to deliver test results when and where they are needed — so people can either get back to work or quarantine themselves — has emerged as a key to re-opening global economies. We are responding to this need by developing a second test that can be produced in much larger quantities than our first, and run on a much larger installed base of instruments.”
In March this year, Hologic secured EUA status for its new Panther Fusion SARS-CoV-2 assay, a real-time RT-PCR in vitro diagnostic test.






