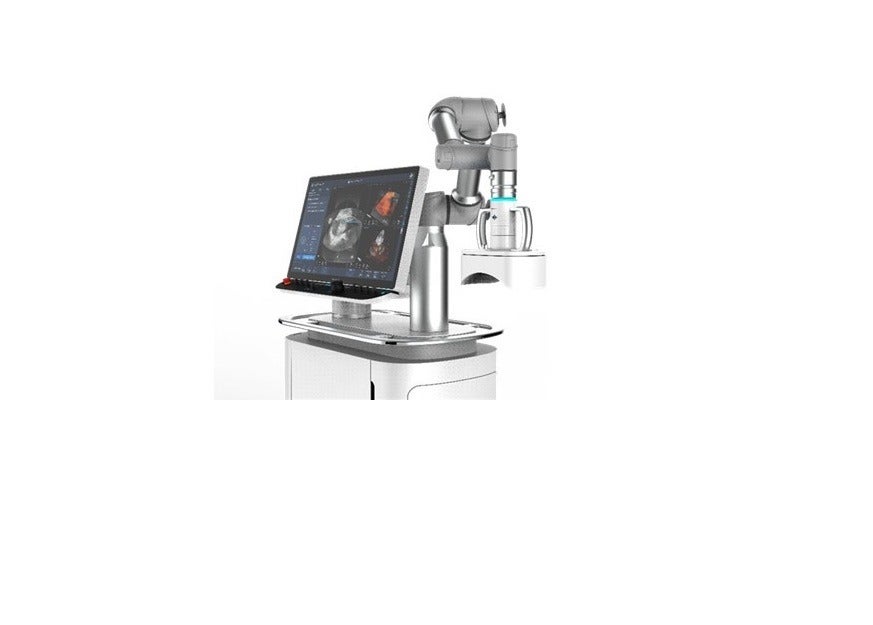
HistoSonics has selected GE Healthcare’s LOGIQ E10 Series ultrasound imaging platform for the real-time visualisation of its novel sonic beam therapy.
Histotripsy is engaged in developing a non-invasive platform and a unique sonic beam therapy using the science of histotripsy to mechanically destroy and liquify unwanted tissue and tumours.
LOGIQ E10 Series is said to be the most technologically advanced ultrasound platform currently available for guiding radiology interventions.
Under the partnership, HistoSonics will distribute GE Healthcare’s LOGIQ E10 Series with its advanced liver therapy system, dubbed Edison, upon receiving market authorisation.
Currently, under development, HistoSonics’ Edison system leverages histotripsy technology to non-invasively destroy targeted liver tissue.
The company intends to use LOGIQ E10 to provide continuous visualisation for unique elements of the histotripsy therapy procedure, which include planning, monitoring, and post-treatment verification.
HistoSonics said that the current agreement with GE Healthcare is aimed at supporting its efforts to roll out the Edison system.
The company is currently enrolling participants in US and Europe, for the #HOPE4LIVER trials, evaluating the safety and efficacy of histotripsy for the destruction of targeted primary or metastatic liver tumours.
Furthermore, it has recently secured the US Food and Drug Administration (FDA) Breakthrough Device Designation for histotripsy of liver tissue.
HistoSonics R&D vice president Josh Stopek said: “We are very excited to formalize our imaging partnership with GE Healthcare, which is a key part of bringing our transformative therapy platform, and an entirely new treatment option, to the clinic and to patients.
“We’ve developed a very collaborative relationship with GE Healthcare and look forward to expanding our efforts to realise the full potential of histotripsy across clinical applications, specialities, and care settings.”






