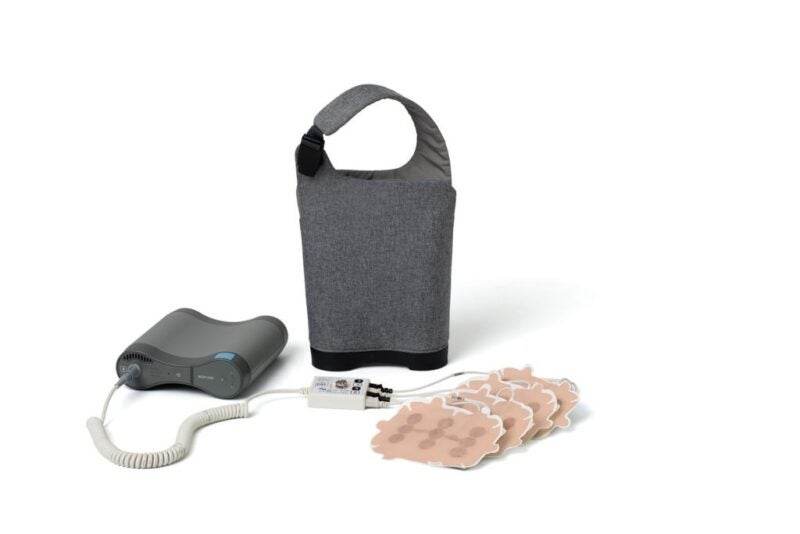
Switzerland-based oncology firm Novocure has received approval from Health Canada for its Optune device designed for the treatment of newly diagnosed and recurrent glioblastoma (GBM).
Optune is said to be a non-invasive, antimitotic cancer treatment which by generating tumour treating fields (TTFields) disrupts cancer cell division. The device sends the electric fields to the region of the tumour.
Weighing 2.7 pounds, Optune is wearable and portable too. It helps to receive continuous treatment from almost anywhere, Novocure said.
The oncology firm claimed that the device has treated over 25,000 patients around the world to date.
Novocure Canada country manager Jovan Antunovic said: “We’re very happy that Health Canada has approved Optune for the treatment of GBM.
“We are grateful for the rapid and diligent review of our submission and for Health Canada’s approval of Optune. This is an important achievement in order to bring our therapy to more patients throughout Canada who can benefit.”
To validate the device, Novocure conducted a phase 3 EF-14 trial that compared Optune plus temozolomide to only temozolomide in 695 patients with newly diagnosed glioblastoma.
According to the firm, overall survival was 20.9 months for patients treated with Optune and temozolomide, compared to 16 months for those treated with temozolomide alone. When combined with temozolomide, Optune was used safely with no appreciable increase in major adverse events compared to temozolomide alone.
Optune is marketed as a glioblastoma treatment in various countries across North America, Europe, and Asia.
Additionally, the oncology firm is working to secure reimbursement for Optune to make the therapy accessible for more glioblastoma patients in Canada.
Novocure chief commercial officer Pritesh Shah said: “We are working to ensure that both public and private payers provide access and full reimbursement for Optune as soon as possible.
“We are proud to have reached this milestone as a company and are committed to making our therapy available to all the patients who may benefit throughout the world.”






