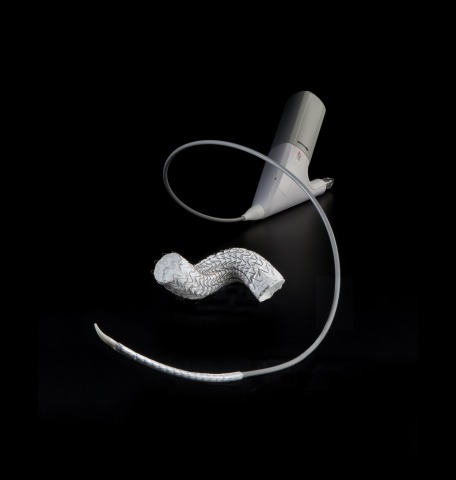
Gore Tag conformable thoracic stent graft with Active Control system is part of the firm’s endovascular product portfolio, which also includes Gore Excluder AAA endoprosthesis to treat abdominal aortic aneurysms (AAA).
The new system is said to be a unique thoracic endovascular aortic repair (TEVAR) solution that combines new levels of control with the proven performance of the conformable Gore Tag device.
Gore device and delivery system is expected to offer new precision and predictable patient outcomes in the endovascular repair of aneurysms, transections, and Type B dissections of the descending thoracic aorta.
A smaller-diameter primary delivery sleeve permits the device and system to access a lower profile across 10 device sizes.
Gore Active Control system offers controlled and two-stage deployment, with primary deployment to an intermediate diameter and a secondary deployment to full diameter.
The design of the device enables continuous blood flow across the deployment with multiple chanced to visualize and refine graft placement. It can also be used for angulation of the proximal end of the device for enhanced seal and apposition.
Large device oversizing windows have been designed and tested to accommodate differences in proximal and distal landing zone diameters.
A unique 6% to 33% oversizing window enables physicians to select the optimal radial force to fit patient anatomy and etiology, helping to treat a young trauma patient or a fragile dissected aorta.
The 16mm to 42mm range will be treated with as few as five sizes, enabling providers to stock fewer devices while treating a broader range of patients.
Gore vascular business leader Eric Zacharias said: “The GORE TAG Device family has a legacy of trusted performance and durability, and we knew we could build on that by enhancing control during deployment, which would help make TEVAR procedures more predictable for physicians.
“Physicians can now deploy our thoracic stent graft in the descending thoracic aorta with more operative ease, even in those patients with challenging angulated aortic arches, and meet the clinical and practical challenges of TEVAR with confidence.”






