FX Shoulder USA has received the US Food and Drug Administration (FDA) 510k approval for its Titanium Nitride (TiN) coated Humeral Heads and Glenospheres.
The new TiN coated humeral heads and glenospheres form the latest shoulder implants to join the company’s portfolio of unique-to-market prostheses.
FX Shoulder USA CEO Baptiste Martin said: “We are now able to reach a broad market and provide surgeons even more shoulder arthroplasty solutions to address patients’ needs. As we continue to expand our portfolio, we truly have one of the more comprehensive portfolios dedicated exclusively to shoulder arthroplasty.”
FX conducted wear testing under worst case load and worst-case environment
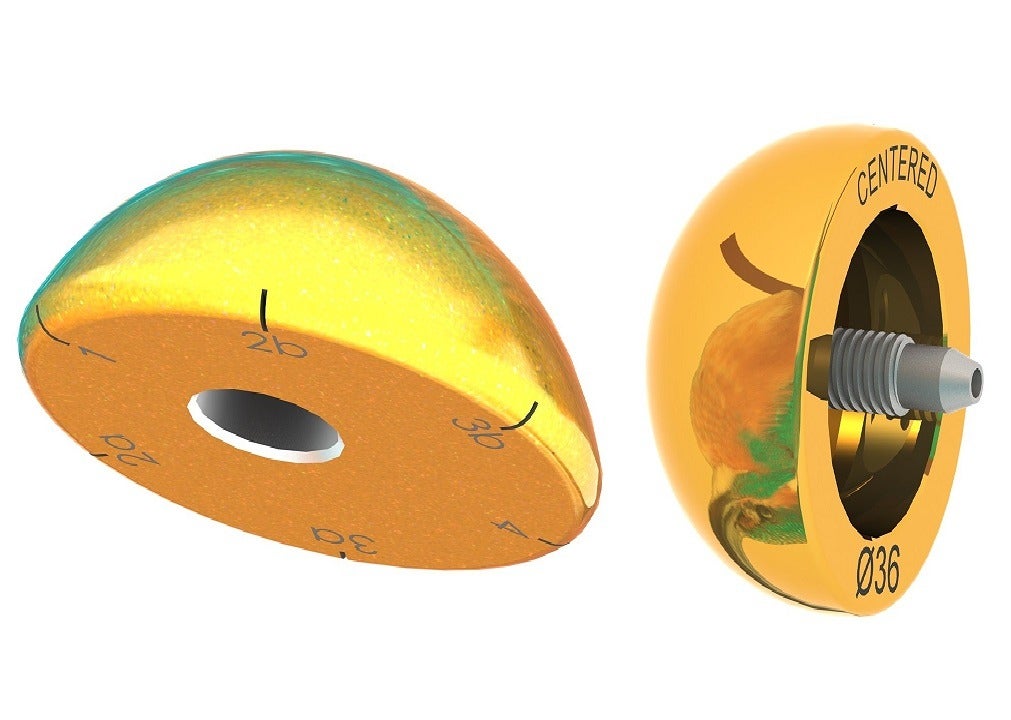
The new TiN coated humeral heads and glenospheres resemble the humeral heads and glenospheres currently offered by the company, but comes with the TiN coating.
The TiN coated humeral head is compatible with its Humelock II and Humeris shoulder products in the anatomic construct while the TiN coated glenosphere is compatible with the Humelock II, Humelock Reversed, and Humeris shoulder systems in the reverse construct.
FX said that the new products have been wear tested under worst case load and worst-case environment, and showed equivalent performance with the uncoated (Cobalt Chromium) products.
Tests for other characteristics demonstrated no difference in results for those with TiN coating than the uncoated product, and there were no TiN particles detected in the analysed test fluids.
Established in in January 2018, FX Shoulder USA is the direct provider of FX Solutions shoulder replacement devices in the US.






