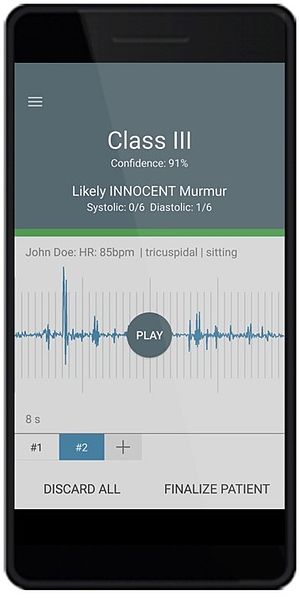
eMurmur ID is a mobile and cloud solution which operates in conjunction with a 3rd party electronic stethoscope. It uses advanced machine learning to identify and classify pathologic and innocent heart murmurs, the absence of a heart murmur, and S1, S2 heart sounds. The end-to-end solution is comprised of AI-based analytics, a mobile app, and a web portal (all HIPAA compliant). It supports the workflows of healthcare providers performing cardiac auscultation and has multiple applications including primary and specialty care, and corporate health.
“The eMurmur ID solution has the potential to disrupt medical care”, said Dr. Derek Exner, Professor and Canada Research Chair in Cardiovascular Clinical Trials at the University of Calgary. “The evidence for its utility is solid and includes five studies on over 1000 patients, including blinded, multi-centre trials. This system has been shown to be accurate, informative, and easy-to-use. It provides healthcare professionals with a potent screening tool and method to confirm their clinical diagnoses, enhancing patient care.”
“Receiving FDA clearance marks a significant milestone in our company’s history, and I’m very excited to finally be able to take the eMurmur ID solution to market,” said Andreas Schriefl, co-founder and CEO of eMurmur. “We believe that eMurmur ID has the potential to disrupt the status-quo of heart murmur screening and significantly reduce costs to healthcare systems worldwide.”
“From a technology perspective, receiving FDA clearance requires solid clinical proof of the algorithm performance characteristics, as well as a quality and risk management system that ensures patient safety and compliance with stringent regulatory standards,” said Andreas Reinisch, co-founder and CTO of eMurmur. “Through this process, our company has gained significant knowledge and expertise to facilitate and support future AI- based medical solutions.”
Source: Company Press Release






