China-based medical device company EndoFresh has received the US Food and Drug Administration (FDA) 510(k) approval for its Disposable Digestive Endoscopy System.
The FDA approval follows multiple reports of persistent cross-contamination among multi-patients by the difficult-to-clean devices and makes the devices safe without reprocessing.
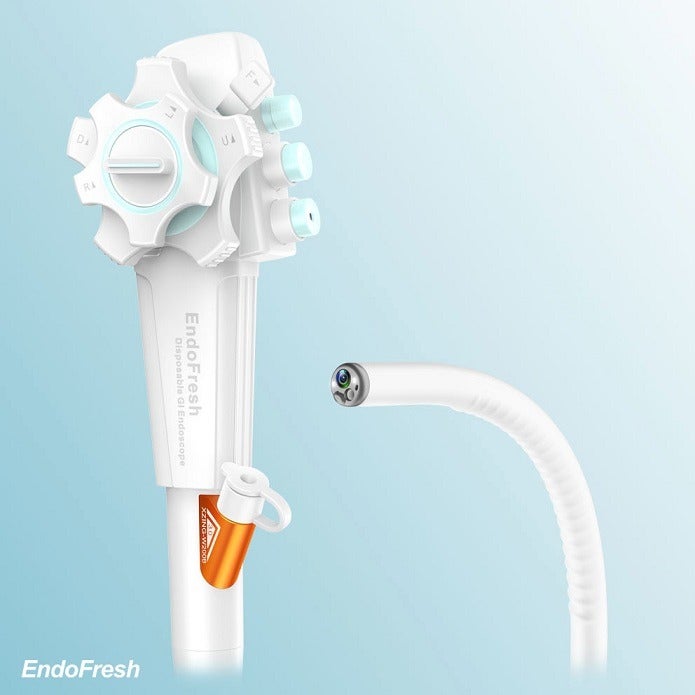
EndoFresh Disposable Digestive Endoscopy System features a camera system with an advanced all-in-one design, disposable upper GI endoscope and disposable colonoscope.
The endoscope and colonoscope are used together with the medical display and other peripheral devices to help physicians visualise, diagnose and operate GI endoscopy.
EndoFresh CEO Lee said: “With this novel system, medical practitioners could offer patients a secure experience, which is available at any time and anywhere.
“It helps to prevent the risk of cross-infection and minimize the workload in preoperative screening and postoperative disinfection.”
Recently, the US regulatory agency has alerted health care providers to the risk of infections associated with reprocessed urological endoscopes.
It has cited more than 450 medical device reports since 2017, describing post-procedure patient infections or other possible contamination.
According to the FDA statement, the issues identified with reprocessed urological endoscopes and duodenoscopes are applicable to similar devices.
Manufacturers are researching and developing disposable endoscope for their safety and effectiveness, as the global flexible endoscope market has been expanding.
EndoFresh said that its advanced technology will address the traditional challenge of expanding endoscopy procedures while making its single-use devices cost-effective, risk-controllable and accessible.
Reusable endoscopes are often associated with high expenses on acquisition and repair, washing equipment and cleaning personnel along with multiple points of failure associated with improper high-level disinfection practices.
Disposable endoscope comes with a risk factor much lower or even negligible, compared to the Reusable endoscopes, said the company.
EndoFresh claimed that it differentiates its devices from traditional reprocessing and time-consuming maintenance solutions and follows strict reliability and quality standards.






