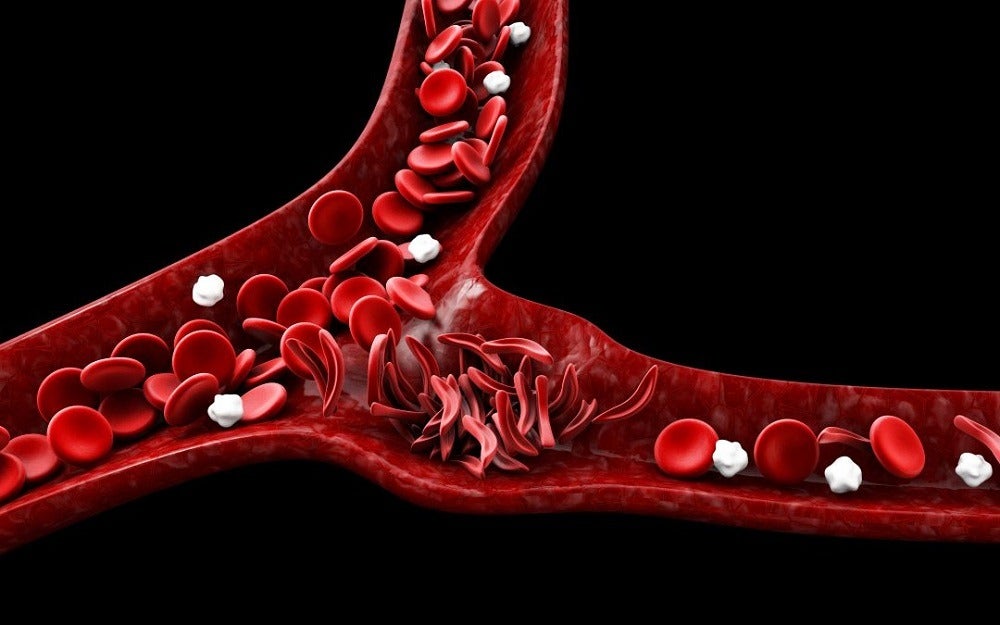
New drugs to treat sickle cell disease (SCD) could be developed with the help of a new computer model that simulates the way red blood cells become misshapen by disease.
The, discovery from Brown University researchers in the US, could identity drugs to treat the killer hemoglobin dysfunction, as it can be applied in the preclinical evaluation of drugs aimed at preventing the sickling process.
World Health Organisation estimates suggest more than 70% of SCD-related deaths are preventable with simple, cost-effective interventions, such as early screening followed by affordable and widely available treatment regimens.
Lu Lu, a PhD student in the Division of Applied Mathematics at Brown and the study’s co-lead author, said: “There are currently only two drugs approved by the FDA for treating sickle cell disease, and they don’t work for everyone.
“We wanted to build a model that considers the entire sickling process and could be used to quickly and inexpensively pre-screen new drug candidates.”
How the computer model works to test new sickle cell drugs?
SCD results from an inherited disorder that leads to red blood cells developing abnormally, distorting their natural disc shape into an abnormal “sickle” shape, which affects the cell’s ability to carry oxygen and move around the body.
Common symptoms include intense pain and severe anaemia, along with damage to major organs and infections, leading to life-threatening complications.
Pain episodes are unpredictable and patients never know when or where these episodes will take place.
The new model combines and simplifies previous models to create a single kinetic model of the entire sickling process.
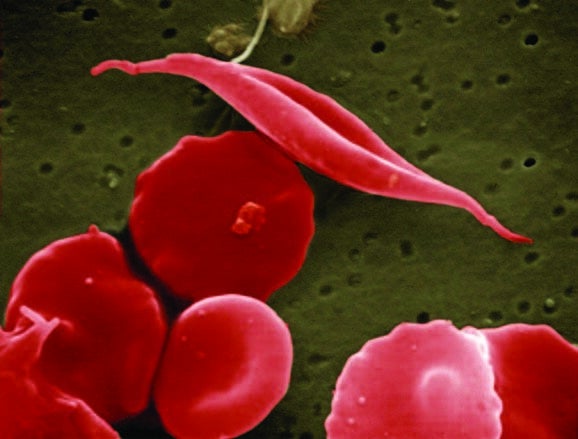
Using information obtained from the detailed supercomputer models, the researchers were able to build a simplified version that captures all the important dynamics of the sickling process, which can also be run on a laptop.
To validate the model, the researchers showed that it could reproduce the outcomes of prior experiments in the lab and in patients.
The model described in a paper titled Quantitative prediction of erythrocyte sickling for the development of advanced sickle cell therapies is published in an open-access scientific journal Science Advances.
The research team designed the model to simulate sickling process in different organs due to the dynamics of the sickling process varying depending on where in the body it’s happening.
For example, because oxygen plays a key role in the process, sickling unfolds very differently in oxygen-rich areas such as the lungs compared to areas like the kidneys.
The model enables users to input parameters specific to the organ they’re attempting to simulate.
George Karniadakis, a professor of applied mathematics at Brown and senior author of the new research, explained that the same flexibility enables a model to be run for individual patients who may have more or less severe versions of the disorder.
To test the potential effectiveness of drugs, the model allows users to input the mode of action by which a drug is presumed to work, information which is often gathered during preliminary lab studies.
Professor Karniadakis said: “Sometimes a drug can be designed to work on one parameter, but ends up having a different effects on other parameters.
“The model can tell if those effects are synergistic or whether they may negate each other. So the model can give us an idea of the overall effect of the drug.
“Clinical drug trials are very expensive and the vast majority of them are unsuccessful,” Karniadakis said.
“The hope here is that we can do in silicon trials to screen potential medications before proceeding to a clinical trial.”






