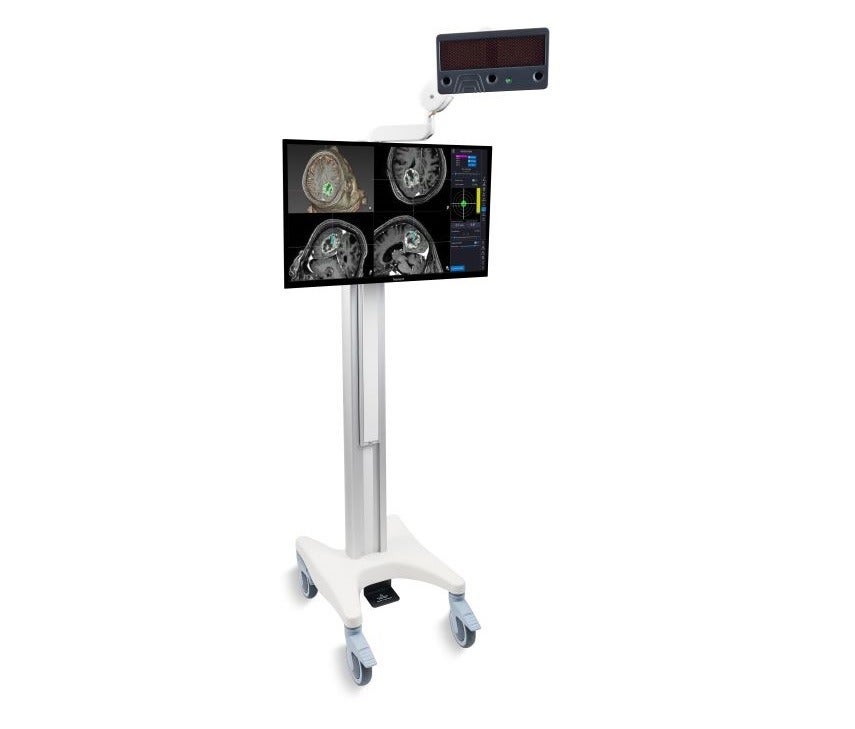
Medical device hardware and software company ClaroNav has secured Health Canada approval for its Navient image guided navigation system.
Through its CKI subsidiary, ClaroNav has received the regulatory approval for its Navient ENT and Navient Cranial as class III medical devices. Earlier, both devices were approved as class II devices.
The company has designed Navient Cranial for trans-cranial procedures, while Navient ENT for trans-nasal surgeries.
Similar to GPS, Navient is a computer-assisted surgical navigation system that helps the surgeon to navigate inside the patient skill.
Designed to offer three-dimensional visualisation, the system enables surgeons to find their ways inside the patient sinuses and prevent critical structures such as the optic nerve and brain, while safely navigating their way to the diseased area.
CKI CEO Ahmad Kolahi said: “This updated clearance is critical for Navient systems that are used for critical life enhancing surgeries.
“Regulatory agencies worldwide continue to focus on improving patient safety and at ClaroNav, we always strive for maximum compliance. With this additional clearance, Navient is now cleared in over 20 countries supporting our global network of distributors.”
Surgical navigation system, which is a standard of care for cranial procedure, facilitates minimally invasive surgery to improve surgical accuracy, reduces tissue damage and recovery time.
According to the company, more than two million brain mass removal is performed per annum and around 29 million Americans are affected by the chronic form of the rhinosinusitis disease with annual direct cost of more than $4bn.
In February this year, ClaroNav Kolahi received Chinese regulatory clearance to market and sell its Navient ENT product in China.






