Centinel Spine, a privately-held spine company, announced that an Investigational Device Exemption (IDE) study, under the Food and Drug Administration (FDA) supervision has completed the first implantation.
The IDE clinical trial is a prospective, randomised, multi-centered clinical study to evaluate two different cervical total disc replacement (TDR) devices at different sites across the US.
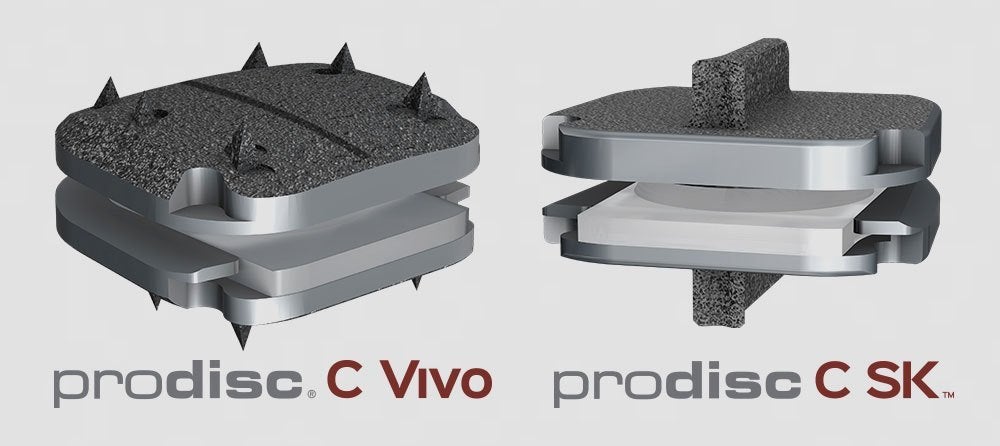
Centinel Spine said that the ongoing IDE clinical trial compares its prodisc C Vivo and prodisc C SK devices with an approved TDR product as a control, to validate the safety and effectiveness of devices, and various centres with experience in TDR procedures are participating in the trial.
DFW Center for Spinal Disorders founder and orthopaedic spine surgeon Jason Tinley said: “This case was the perfect justification of the intent behind the design of these devices. The patient had severe neck pain and radiating neurologic symptoms, associated with disc degeneration and spinal stenosis that worsened despite months of non-operative treatment.
“I was able to select the most stable and least invasive implant that best suited the patient’s anatomy, providing me with a significant advantage as I seek to optimize patient clinical results and preserve spinal motion with arthroplasty.”
Centinel Spine offering prodisc C Vivo product since 2009
The company has been offering prodisc C Vivo product since 2009, which marks the most frequently implanted TDR product outside of the US, along with prodisc C SK implant, a variation of the prodisc C Nova device, since 2010.
Both the products prodisc C Vivo and prodisc C SK, would deploy the same mechanism as in prodisc C implant, which secured premarket approval (PMA) approval and used across the US.
The two products are different from prodisc C in interface with the vertebral body endplates, and the interface variations will allow surgeons to better match the implant to a patient’s anatomy.
Centinel Spine chairman & CEO John Viscogliosi said: “The first study implantation is an important first step towards obtaining sales and marketing approval of the prodisc C Vivo and prodisc C SK products in the United States.
“The prodisc platform is already the most clinically studied and proven TDR technology in the world with more than 450 papers, and this study will add to the body of evidence. The goal of Centinel Spine is to provide surgeons with multiple clinically proven TDR implant options to match a patient’s anatomy.”






