Medical technology company Boston Scientific has secured approval from the US Food and Drug Administration (FDA) for its Ranger drug-coated balloon (DCB).
The company has developed Ranger DCB to treat patients with peripheral artery disease (PAD) in the superficial femoral artery (SFA) and proximal popliteal artery (PPA).
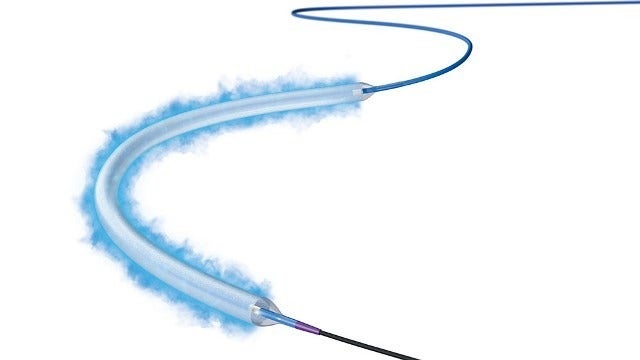
With a low therapeutic drug dose and an advanced coating, the Ranger DCB enables to efficiently transfer the drug into the tissue. It facilitates high primary patency rates and low systemic drug exposure for patients.
Ranger DCB’s low-profile platform helps clinicians to conduct streamlined procedures
The low-profile platform of the balloon also enables clinicians to conduct streamlined procedures and navigate through challenging anatomy to deliver consistent therapy.
The FDA approval was based on data from the RANGER II SFA pivotal study, which assessed the safety and effectiveness of the Ranger DCB against standard percutaneous transluminal angioplasty (PTA) to treat patients with PAD in the SFA and PPA.
According to the company, the Ranger DCB also showed up to 90% primary patency in the investigator-sponsored COMPARE trial. It is the first head-to-head prospective and randomised controlled study that compared two different DCBs.
In 2014, the company secured CE mark approval for the Ranger DCB. Boston Scientific intends to immediately introduce the device in the US.
Boston Scientific peripheral interventions president Jeff Mirviss said: “This approval allows us to bring more treatment options with exceptional outcomes and proven safety to U.S. physicians and their patients who are facing this challenging disease.
“Adding the Ranger DCB to our drug-eluting portfolio, which also includes our Eluvia Drug-Eluting Vascular Stent System, reinforces our commitment to providing differentiated technology with strong clinical evidence that supports data-driven treatment decisions for millions of patients suffering from PAD worldwide.”
In July, Boston Scientific secured FDA approval for its WATCHMAN FLX left atrial appendage closure (LAAC) device.
The company has designed the next-generation WATCHMAN FLX device to minimise the risk of stroke in patients with non-valvular atrial fibrillation (NVAF) who require an alternative to oral anticoagulation therapy by permanently closing off the left atrial appendage.






