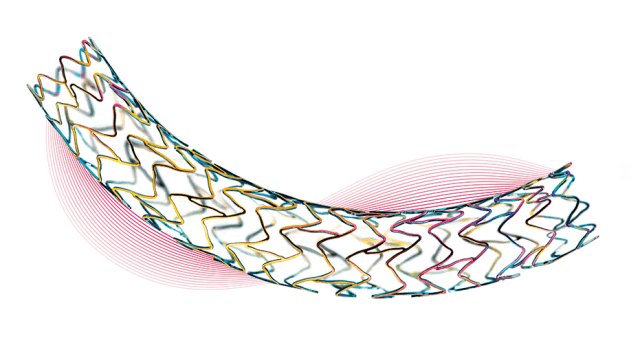
Orsiro, which is claimed to be the first and only ultrathin DES, secured CE mark approval in 2011 and has so far been used to treat over one million patients across the world.
Bioflow-V study showed that Orsiro enabled to significantly reduce target lesion failure (TLF) and target vessel myocardial infarction at 12 months compared to Xience in a large and complex patient population.
Bioflow-V US principal investigator Dr David Kandzari said: “Bioflow-V data are the best clinical outcomes witnessed with modern DES. It was largely thought that efficacy findings were unsurpassable, but Orsiro proves we can further reduce event rates with meaningful innovation.”
For application in percutaneous coronary intervention (PCI) procedures, the cobalt chromium metal stent elutes sirolimus through BIOlut, which is the Biotronik’s bioabsorbable polymer coating.
Below the bioabsorbable layer is Biotronik’s proBIO, which is a passive coating on the bare metal surface. It is designed to reduce nickel ion release.
Orsiro stent system has been developed to offer ultrathin stent struts without disturbing radial strength and low crossing profile for easier lesion cross in complex PC.
The company is providing Orsiro in 52 sizes ranging from 2.25mm to 4.0 mm in diameter and lengths up to 40mm. Biotronik is currently marketing Orsiro stent system in the US.
Biotronik president Ryan Walters said: “The FDA approval of Orsiro changes the dynamic of what had become a highly commoditized DES market.
“We designed Orsiro for use even in challenging cases with features that make it unlike any other DES in the world. Hospital administrators now have available a DES that shows improved clinical event rates and interventionalists can rely on Orsiro’s deliverability to treat complex lesions6 and challenging subgroups to achieve unprecedented patient outcomes.”
Based in Berlin, Biotronik provides cardiovascular and endovascular solutions to the customers in more than100 countries. The firm has US offices in New York City and Lake Oswego of Oregon.






