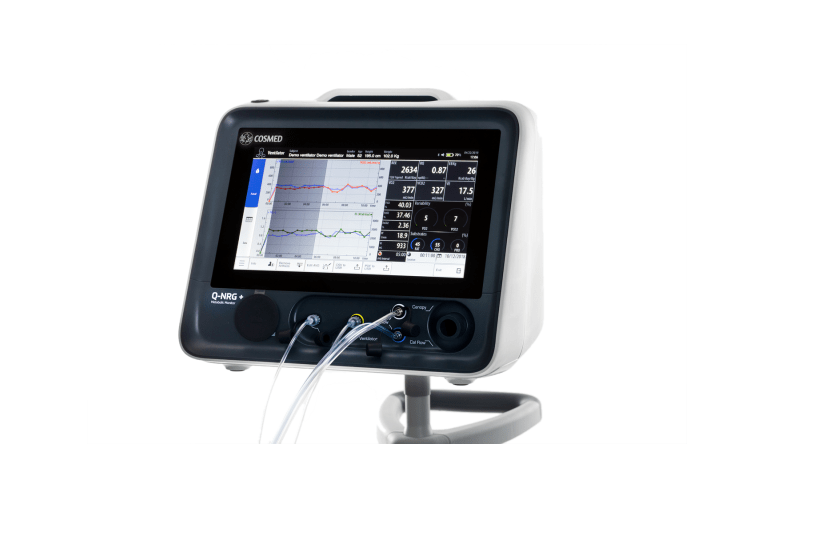
Baxter International, a clinical nutrition firm, has obtained the US Food and Drug Administration (FDA) approval for its metabolic monitoring device Q-NRG+.
The US healthcare firm has partnered with Italian medical technology company COSMED, for the rights to commercialise Q-NRG+ in at least 18 markets across the world, with an option for further expansion.
The FDA approval is the latest regulatory approval for the metabolic monitoring device, which is currently sold across 12 countries in Europe, along with Canada, Australia and New Zealand.
Baxter US hospital products general manager Heather Knight said: “It can be challenging to prescribe clinical nutrition without knowing the exact caloric needs of a patient.
“With Q-NRG+, clinicians will have access to the latest technology to accurately measure energy requirements and won’t have to rely on predictive equations or dated technology, which can be cumbersome and time consuming.”
Q-NRG+ takes least time to warm-up and calibrates automatically between uses
According to the company, traditionally caloric requirements are assessed using predictive equations, based on factors including weight, height, gender and age, which often result in inaccurate measurements and could lead to overfeeding or underfeeding.
Baxter said that the Q-NRG+ leverages indirect calorimetry (IC) technology, which is considered as gold standard for precise measurement of resting energy expenditure (REE), the calorie needs of patient.
The measured REE values are said to help physicians in prescription and administration of nutrition therapy, including parenteral nutrition (PN), the intravenous administration of nutrients.
To offer flexibility and hospital efficiency, the device has been designed as a compact, portable device that can be easily moved between patient rooms. It can be used to test both adult and paediatric patients, along with spontaneously breathing and mechanically ventilated patients.
The Q-NRG+ portable metabolic monitor is intended for use only in professional healthcare facilities. Baxter is planning to commercialise the device in the US in March 2020.






