Medical device company Ancora Heart has recruited the first patient in the CORCINCH-HF pivotal trial of the AccuCinch ventricular restoration system.
The trial has been designed to assess the safety and efficacy of the AccuCinch system in patients who have symptomatic heart failure (HF) with reduced ejection fraction (HFrEF).
Ancora Heart will use the results from the trial to submit a premarket approval (PMA) application to the US Food and Drug Administration (FDA).
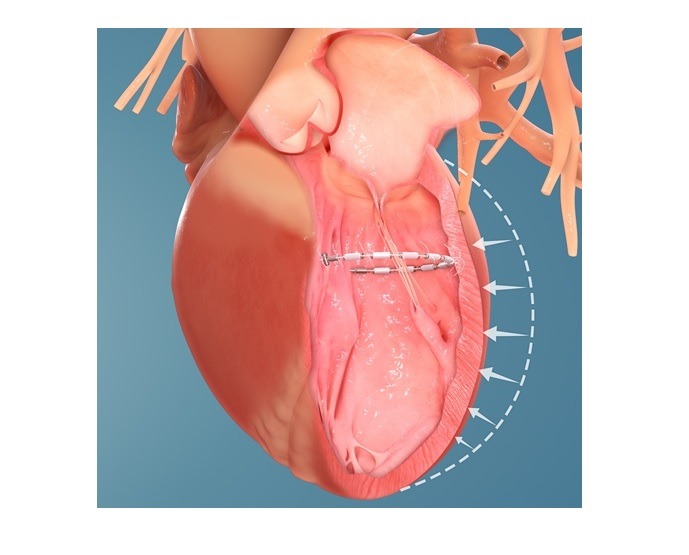
AccuCinch is a device-based therapy
AccuCinch is said to be the only completely transcatheter procedure to treat the enlarged left ventricle. It is a device-based therapy designed to enhance the structure and function of the heart.
The prospective, randomised, open-label, multicentre, international, clinical safety and efficacy study will recruit 400 patients at around 80 centres across the world.
The study design allows Ancora to go for the PMA submission of the initial analysis of safety and clinical efficacy of AccuCinch after the first 250 patients reach six months of follow up.
Later, a second analysis will be submitted after the entire cohort after reaches one year of follow-up.
The AccuCinch system is believed to serve as an enhanced cardiological care for patients in whom HF has progressed beyond the ability of medications and pacemakers to manage symptoms.
It is expected to fill the gap between medication or pacemaker therapy and left ventricular assist devices (LVADs) or a heart transplant.
A flexible implant is attached to the inner wall of the left ventricle and then cinched during the minimally invasive procedure of the AccuCinch system.
The implant is intended to minimise the size of the left ventricle, reduce ventricular wall stress, as well as strengthen the heart wall.
Ancora Heart president and CEO Jeff Closs said: “We look forward to working with our clinical partners to conduct the CORCINCH-HF Study and together move closer to improving treatment options for the millions of people suffering from heart failure.
“Previously collected data on early feasibility implants suggests that the AccuCinch System may afford significant clinical benefits to patients suffering from heart failure with reduced ejection fraction.”
In May 2018, Ancora Heart raised $17.8m financing to support clinical trials of its AccuCinch ventricular repair system.






