Packaging giant Amcor has today launched a speciality dual chamber pouch for drug-device combination products.
Available in Europe for the first time, the pouch has already won a 2021 Award in the US from the Flexible Packaging Association for its technical innovation and material structure.
Combination medical devices demand high performance packaging materials to ensure the sterility and shelf life of the product, with wrappers needing to provide a barrier against light, moisture and oxygen to maintain the stability and efficacy of the active pharmaceutical ingredients (API), while also maintaining sterility until the point of use.
Amcor vice president of R&D for the EMEA region Noemi Bertolino said: “To protect a combination sterile device with a therapeutic drug agent, we knew there were a lot of requirements to fulfil.
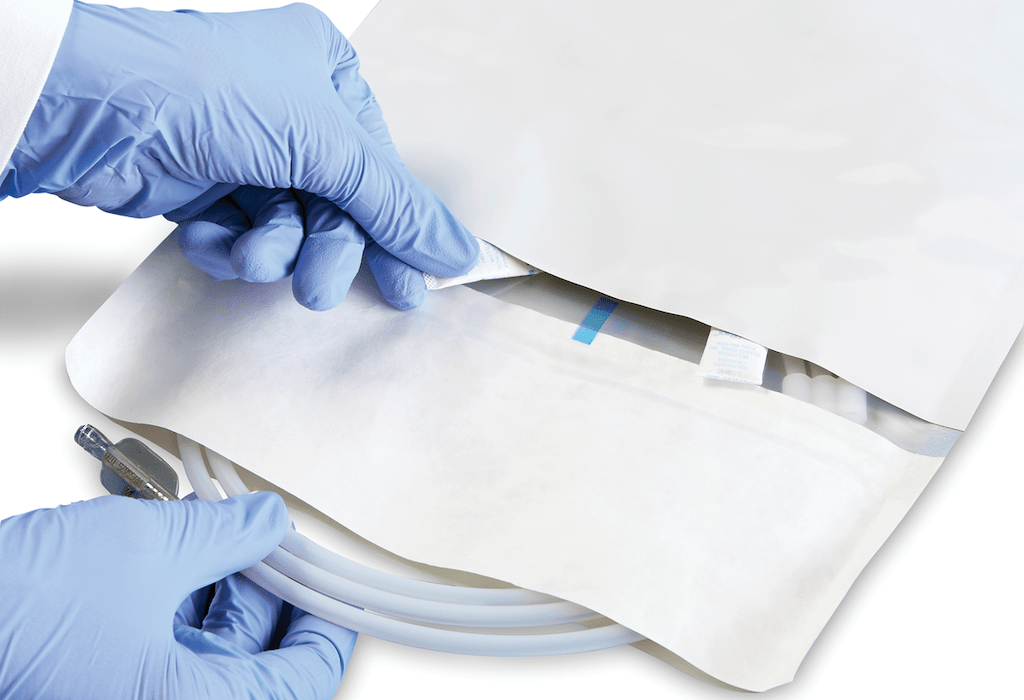
“The first objective was to create a pack design that was easy and intuitive to open and could be aseptically presented.
“The integrity of the drug’s effective dosage also had to be maintained in order to minimise the ingress of moisture vapor into the pouch, so we created a vent towards the centre of the inner film away from the seams.
“This solution maintains shelf life and minimises any risk of contamination. Our Dual Chamber Pouch complies with necessary medical and safety regulations, in an efficient, simplified design.”
How does Amcor dual chamber pouch improve on traditional packaging
The two opposing but critical requirements of ensuring breathability for sterilisation and keeping a barrier in place to maintain ingredient stability pose a real challenge for packaging design.
To overcome this, stents for example, are typically packaged in 2 separate pouches, the first to allow the ETO (ethylene oxide) sterilisation process, and a secondary outer foil pouch with desiccant to preserve drug stability.
Amcor’s innovation is a single, multicompartment pouch with a breathable membrane separating the two chambers, in a safer and simpler design.
The new Amcor dual chamber pouch uses a high-strength foil laminate, that protects from light, moisture and oxygen ingress to support shelf life and drug efficacy.
One side is peelable and allows aseptic presentation and easy access to the device.
The second non-peelable chamber houses the desiccant sachet or other scavenging technologies.
The internal breathable vent allows gas exchange for the desiccant to maintain a controlled environment within the pouch.
The separate chambers eliminate any risk of the desiccant encountering the sterile device and sterile field.
A porous header, that uses DuPont Tyvek is added to the pouch, which provides an easy method of ETO sterilisation, after which the pouch is sealed, the header removed, and the barrier pouch is ready for shipment.
The packaging device will be available in Europe from the company’s healthcare packaging site in Sligo, Ireland.






