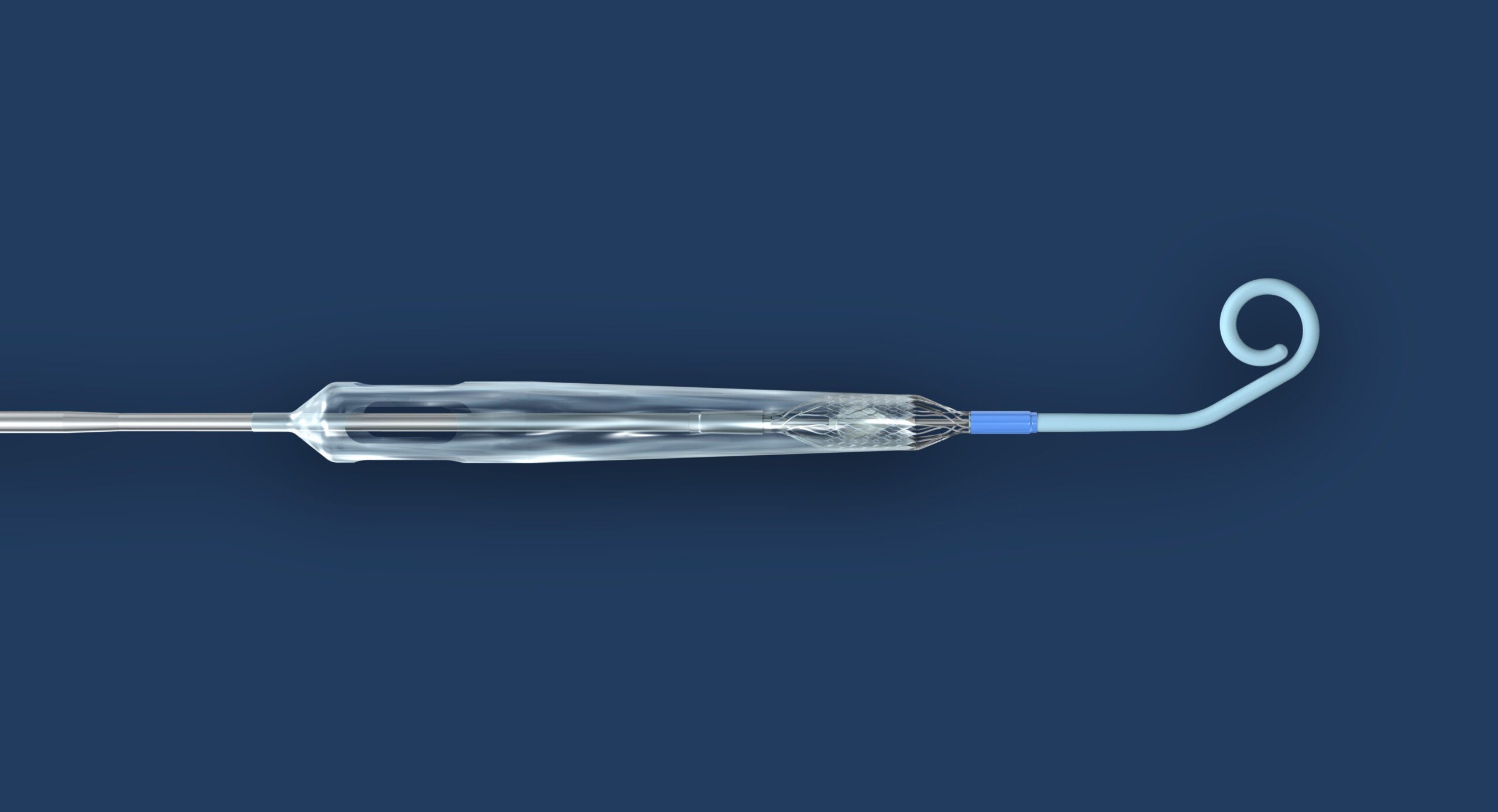
Abiomed announced that the US Food and Drug Administration (FDA) has approved a version of Impella ECP heart pump for use in the company’s upcoming clinical trial.
The medical device maker enrolled the first two patients in the study, which is designed to include up to 217 patients in the US.
Ascension St. John Hospital in Detroit mechanical circulatory support director Amir Kaki is leading the single-arm, multi-centre clinical trial as principal investigator.
In the study, adults undergoing an elective, or urgent high-risk percutaneous coronary intervention (PCI) will receive treatment using Impella ECP.
The study will evaluate the rate of major adverse cardiovascular and cerebrovascular events (MACCE) in the participants.
Kaki said: “The research and clinical teams at Ascension St. John are delighted about enrolling the first patients in the Impella ECP FDA Pivotal Trial.
“Impella ECP advances the opportunity for physicians to provide critical hemodynamic support during high-risk PCI procedures by delivering similar or higher flow compared to other options through a smaller vascular sheath for access.
“This technology has the potential to improve patient safety and cath lab throughput because of the smaller arteriotomy required for pump placement.”
Abiomed said that its Impella ECP is the world’s smallest heart pump and the only heart pump compatible with small bore access and closure techniques.
It is designed to measure 9 Fr in diameter upon insertion and once implanted, it expands to support the heart’s pumping function, enabling peak flows of up to 5L/min.
In the study, both patients received Impella ECP support during challenging left main coronary bifurcation stent procedures involving heavily calcified lesions.
After Impella ECP was removed, the first patient was closed with an 8Fr closure device.
Abiomed chairman, president and chief executive officer Mike Minogue said: “Impella ECP demonstrates Abiomed’s leadership in technology and innovation as we have broken the small-bore barrier through the development of the world’s smallest heart pump.
“At Abiomed, we remain committed to developing smaller, smarter and more connected technologies that will improve outcomes for patients with heart disease.”
In June 2020, Abiomed has received the FDA approval for its Impella ECP early feasibility study (EFS), in which 54 patients have been treated till date.
In August last year, the US health regulator has granted breakthrough device designation Impella ECP expandable percutaneous heart pump.






