UK-based medical device company Terumo Aortic has announced the first commercial implant of the Thoraflex Hybrid device in the US.
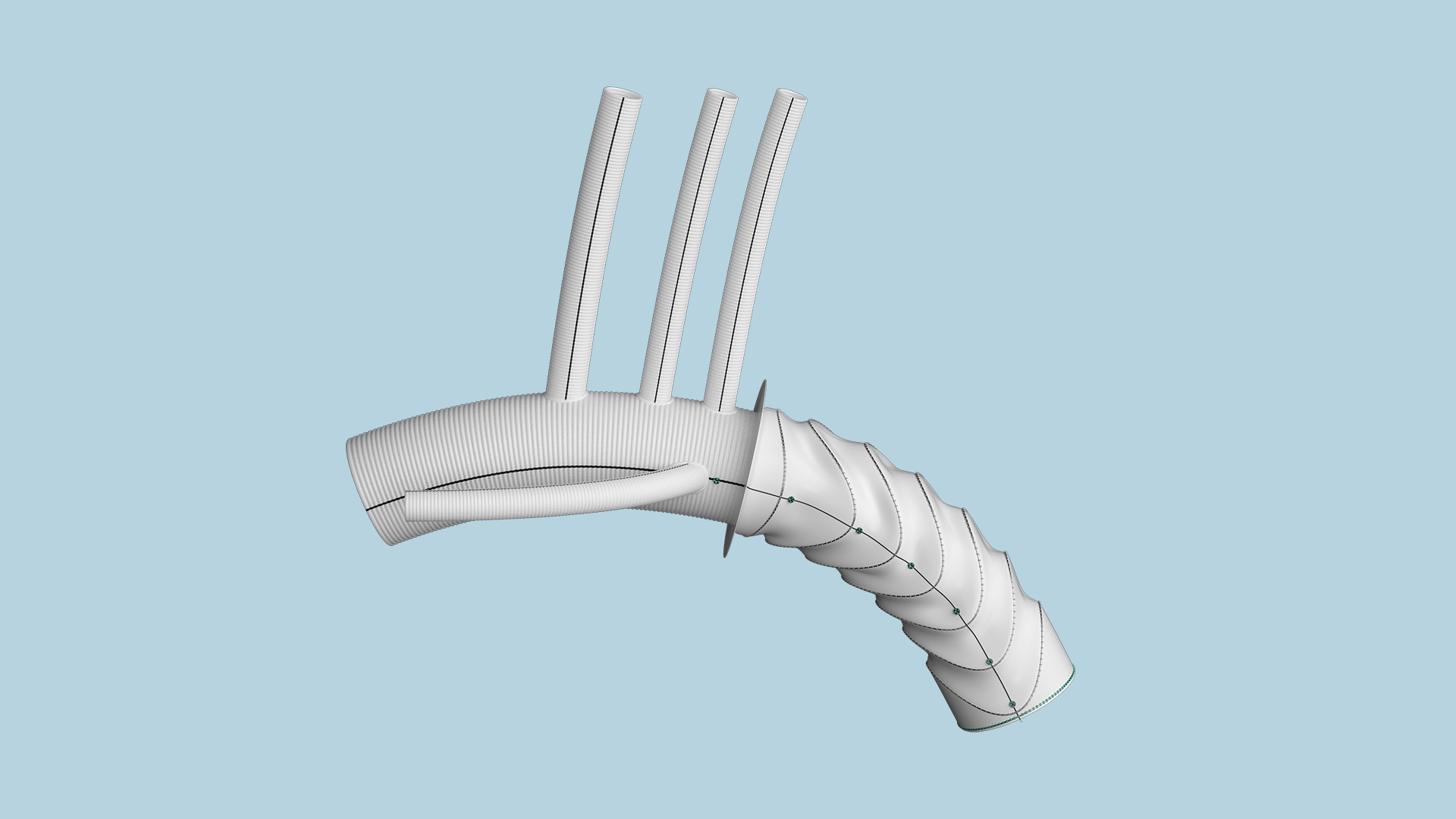
Thoraflex Hybrid is a single-use medical device that combines a Nitinol self-expanding stent-graft with a Gelweave polyester graft.
It is intended for the open surgical repair or replacement of diseased or damaged aortic arch arteries as well as for the repair of the descending thoracic aorta with or without the involvement of the ascending aorta.
The firm received CE Mark approval in 2012 and FDA clearance in 2022 for the device and had sold more than 13,000 devices worldwide over the last 10 years.
The Thoraflex Hybrid study’s lead researcher, Professor Joseph Coselli, executive vice-chair of the Division of Cardiothoracic Surgery at Baylor College of Medicine USA, implanted the patient.
Professor Joseph Coselli said: “The procedure was very successful, the device performed well, and the patient is making a good recovery.
“Thoraflex Hybrid is the first of its kind device used in FET repair in the United States and it will allow US physicians to treat patients who may be at great risk of rupture with a device that brings the primary benefit of requiring a single-stage procedure for those with suitably limited disease, instead of two procedures which has been the conventional pathway in the United States for this group of patients.”
Terumo Aortic North America president Paul Kuznik said: “This commercial implant represents a significant milestone for the company and a tremendous opportunity for Terumo Aortic in the United States.
“This innovative hybrid device complements our open surgical graft and endovascular portfolio making us one of the strongest medical device companies within the aortic space, helping to deliver our commitment to provide solutions for every aorta.”
The company recently received approval from the FDA for Thoraflex Hybrid frozen elephant trunk (FET) device for the treatment of patients with the complex aortic arch disease.






