Reflow Medical has secured breakthrough device designation from the US Food and Drug Administration (FDA) for its Temporary Spur Stent System.
The new retrievable stent technology has been developed to treat below-the-knee (BTK) peripheral artery disease.
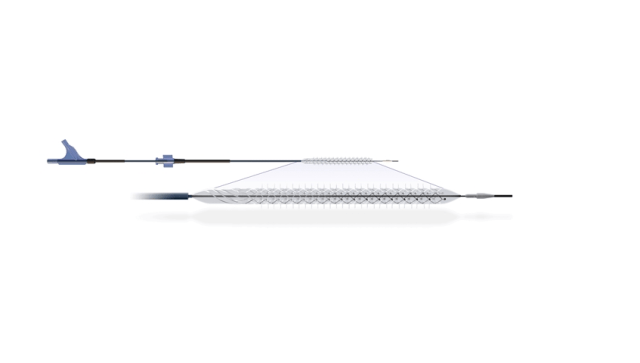
The Temporary Spur Stent System is a new combination device, which includes a patented retrievable stent system with a series of radially expandable spikes.
The spikes have been designed to form various multiple pathways to deliver antiproliferative drugs for elevated uptake into the vessel wall, as well as enable acute luminal gain.
Temporary Spur Stent System will help address treatment challenges in patients with BTK disease
Reflow has developed the device to address high rates of restenosis and treatment challenges in patients with BTK disease.
The Temporary Spur Stent System is an investigational device, and not yet approved for sale anywhere.
The company is also planning to develop the system for use in other clinical areas. It intends to use the Spur technology platform to develop the device for other clinical applications.
Reflow Medical CEO Isa Rizk said: “We are extremely grateful to the FDA for their expedited designation of the Temporary Spur Stent System as a Breakthrough Device.
“We plan to take full advantage of the Program’s benefits, accelerating our efforts towards meeting the requirements of the review process as we advance this novel technology, with the goal of improving the lives of patients.”
In July 2019, Reflow Medical started the DEEPER OUS clinical trial, which is designed to evaluate the Temporary Spur Stent System.
DEEPER OUS is a 100-patient prospective, non-randomised and multicentre trial that will assess the safety and efficacy of the Temporary Spur Stent System compared against meta-analysis of published data for percutaneous transluminal angioplasty (PTA) in treating patients with infrapopliteal disease.
Reflow Medical is engaged in the development of advanced technologies for cardiovascular disease. It is currently developing a family of products for the treatment of cardiovascular disease.






