MicroPort Cardio Rhythm Management (CRM) has enrolled first patients in the Astral-4LV clinical trial, designed to evaluate the safety and efficiency of its advanced quadripolar left ventricular lead Axone.
Axone is designed for use in heart failure patients requiring Cardiac Resynchronisation Therapy (CRT), with the implantation of a CRT pacemaker (CRT-P) or CRT implantable defibrillator (CRT-D).
Astral-4LV is a single arm, multicentre clinical trial, planned to enrol 152 patients at 20 centres in France, Germany, Italy, Netherlands, Portugal, Spain, and Austria.
The clinical study is aimed at evaluating the chronic safety and performance of the Axone lead for CRT.
Related complication free rate and the success rate of left ventricular pacing are the primary objectives of the study, and secondary objective includes the success rate of a bi-zone left ventricular pacing.
The first implantation of the Axone lead as part of the Astral-4LV study was performed at the University Hospital of Rouen, in France, by professor Frédéric Anselme.
Anselme said: “Approximately 30% of patients do not respond to CRT therapy, and one of the main reasons is the difficulty of being able to pace the left ventricle in the correct locations.
“Axone makes it possible to find left ventricular pacing sites that are inaccessible with standard leads. Axone is what we needed to improve the response rate to CRT and have a positive impact on patient outcomes.”
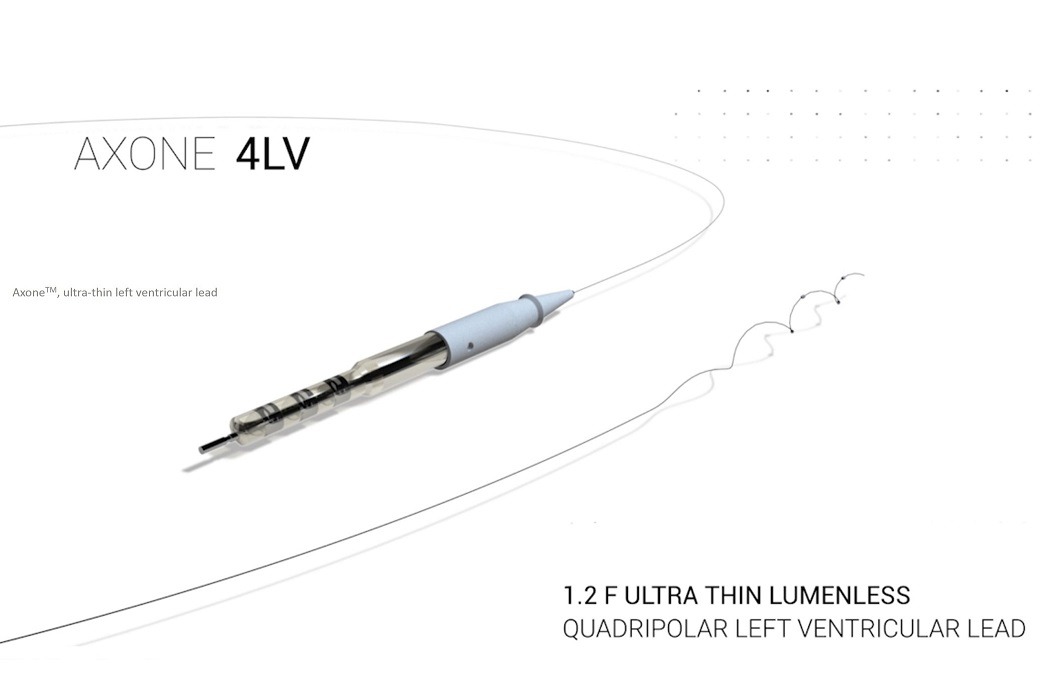
Axone designed for enhanced navigation in narrow coronary veins.
CRT therapy is indicated in heart failure patients with cardiac desynchronisation, and requires placing a pacing lead in the left ventricular (LV) coronary venous system.
MicroPort CRM said that its Axone lead has 0.4mm diameter, which is nearly 4 times smaller than the diameter of a standard LV lead, and is designed to navigate effectively in narrow and tortuous coronary veins.
Also, Axone features four widely-spaced electrodes to provide enhanced options for LV pacing sites and facilitate multipoint pacing with distant electrodes (bi-zone pacing), for wider resynchronization.
The company is leading the research project in collaboration with Heraeus, the University Hospital of Rouen in France, and Maastricht University in Netherlands.
Furthermore, the European Union’s Horizon 2020 research and innovation programme has offered funding support for the Axone Project. Data from the Astral-4LV study will be used to support CE marking of Axone.
MicroPort CRM president Benoît Clinchamps said: “We continue to invest in innovative technologies and support clinical trials that provide the strongest possible evidence of safety and efficacy for our therapies.
“The Axone lead and Astral-4LV clinical study highlight our commient to improving the quality of life of patients and saving more lives. We strongly believe that Axone has the potential to be a major breakthrough in the treaent of heart failure through resynchronization therapy.”






