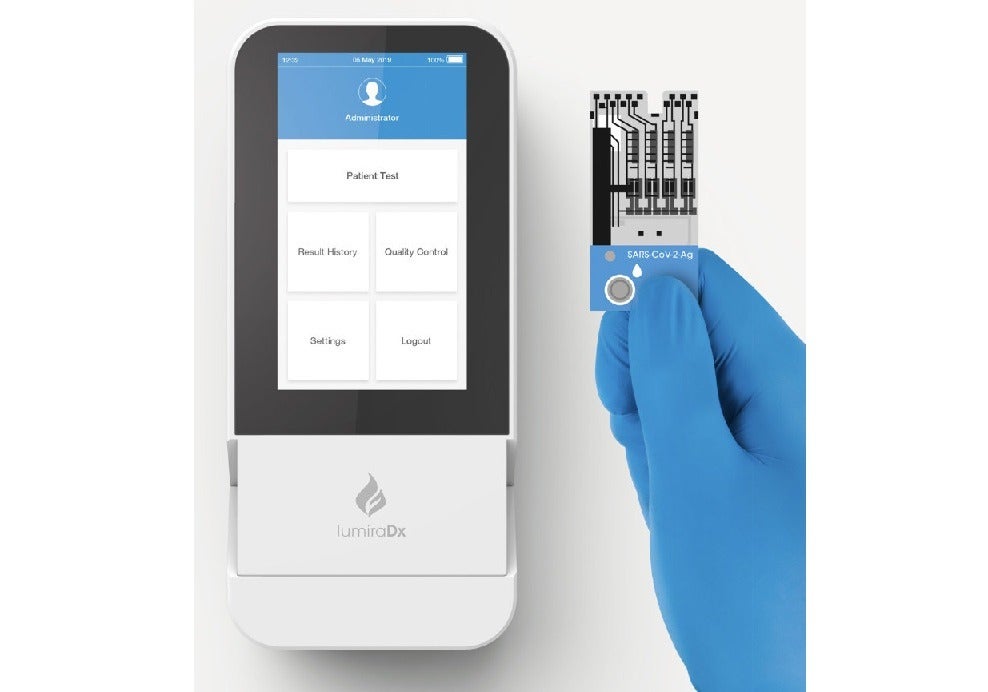
LumiraDx has secured emergency use approval from India’s Central Drugs Standard Control Organisation for its SARS-CoV-2 Antigen test for use in India.
The test is already available in the US after it obtained emergency use authorisation (EUA) from the Food and Drug Administration (FDA) in August this year.
In September, the LumiraDx SARS-CoV-2 Antigen test received CE mark in Europe.
The test is designed to detect antigen nucleocapsid protein from a nasal swab and deliver results in under 12 minutes from sample application.
Clinical studies conducted to evaluate the SARS-CoV-2 Antigen test have revealed 97.6% positive agreement and 96.6% negative agreement with the PCR test for patients within the first twelve days of symptom.
LumiraDx chief commercial officer David Walton said: “The mission of LumiraDx is to transform community-based healthcare through POC diagnostics and make lab-comparable tests accessible to all.
“Our launch in India with our SARS-CoV-2 Antigen test is an important step forward in this mission. We are proud to now have a presence in one of the world’s fastest-growing economies and have the opportunity to partner with local health systems and businesses across the country to provide highly accurate and rapid testing.”
The LumiraDx SARS-CoV-2 Antigen test is a microfluidic test that is developed to run on the LumiraDx point of care platform.
To deliver lab-comparable diagnostic tests on a single point of care instrument, the platform integrates techniques used in laboratory analysers.
The platform features a small, portable instrument, microfluidic test strip and digital connectivity to the cloud and hospital IT systems.
Currently, LumiraDx has five tests on the market including a portfolio of COVID-19 testing solutions, as well as its INR and D-Dimer tests.
The company is also developing tests for Covid/Flu, CRP, HbA1c, high sensitivity troponin I, and Tuberculosis (TB).






