Becton, Dickinson and Company (BD) has secured CE mark approval for its portable and rapid point-of-care SARS-CoV-2 antigen test.
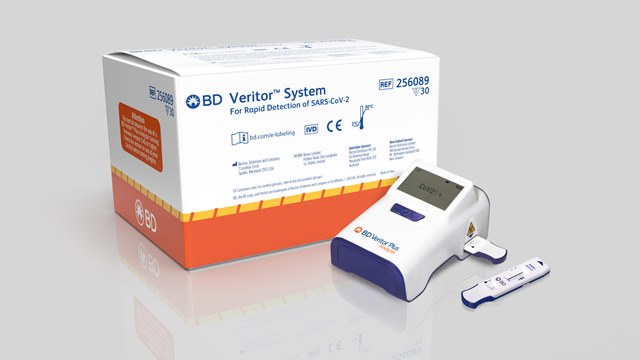
The test, which will be carried out on the BD Veritor plus system, has the capacity to detect SARS-CoV-2 in 15 minutes.
The BD Veritor plus system for rapid detection of SARS-CoV-2 Assay was authorised to identify proteins from SARS-CoV-2 and not for any other viruses or pathogens.
BD will begin the commercial availability of the new assay by the end of this month in countries accepting the CE mark.
The rapid point-of-care SARS-CoV-2 antigen test already secured EUA status from FDA
Since July, the rapid point-of-care SARS-CoV-2 antigen test has been available in the US via an emergency use authorisation (EUA) by the US Food and Drug Administration (FDA).
The BD Veritor plus system is widely used across Europe to test for conditions such as Group A Strep, influenza A+B and Respiratory Syncytial Virus (RSV).
With a size of larger than a mobile phone, the BD Veritor plus system delivers an easy-to-use workflow and is a suitable solution for point-of-care settings.
By using an optional BD Synapsys informatics solution, the system also delivers traceability and reporting capabilities to the customers. It holds the capacity to test for both infections on the same platform.
BD EMEA region president Roland Goette said: “Availability of the SARS-CoV-2 assay on the BD Veritor Plus System in Europe builds on our molecular test on the BD MAX™ System that has been available since March.
“The addition of a truly portable, point-of-care test that can deliver results while the patient waits will be welcomed by health care providers and patients alike to help protect against additional waves of Covid-19.”
In July this year, BD received a $24m investment from the US government to ramp-up the manufacturing of its BD Veritor Solution for rapid detection of SARS-CoV-2.






