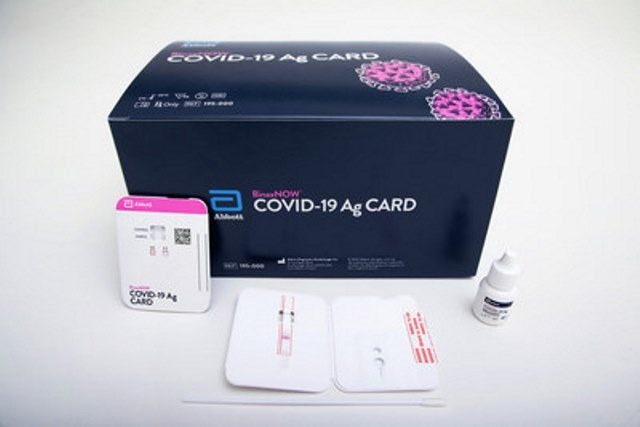
Abbott has secured emergency use authorisation (EUA) from the US Food and Drug Administration (FDA) for its BinaxNOW Covid-19 Ag Card rapid test.
BinaxNOW COVID-19 Ag Card test is suitable for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in nasal swabs from individuals suspected of Covid-19 by their healthcare provider within the first seven days of symptom onset.
Abbott’s easy-to-use test delivers results in 15 minutes
The highly portable test, which will be available at the cost of $5, has been designed to deliver results in 15 minutes.
A simple nasal swab will be used for the collection of specimens from people suspected of having an active infection.
The test will not use any equipment to process samples or read results. It just uses minimal chemical reagents, enabling to reduce exposure to biohazardous materials.
Abbott lateral flow technology will allow to use the rapid test for frequent mass testing through the healthcare provider.
The EUA status enables healthcare professionals to use the near-person rapid antigen test in point-of-care settings. Doctors, nurses, school nurses, medical assistants and technicians, pharmacists, and employer occupational health specialists can conduct the test within these settings.
The company will also introduce a complementary mobile app, dubbed NAVICA, which will help people to show their results secured from a healthcare provider when entering facilities requiring proof of testing
Abbott said that the BinaxNOW COVID-19 Ag Card is the company’s sixth test available for the novel coronavirus detection in the US.
Abbott president and CEO Robert Ford said: “We intentionally designed the BinaxNOW test and NAVICA app so we could offer a comprehensive testing solution to help Americans feel more confident about their health and lives.
“BinaxNOW and the NAVICA app give us an affordable, easy-to-use, scalable test, and a complementary digital health tool to help us have a bit more normalcy in our daily lives.”
In May, Abbott secured FDA EUA status for its SARS-CoV-2 IgG lab-based serology blood test on the Alinity i system.






