Responding to the worsening problem of poor quality medical devices resulting in frequent recalls in the US, SoomSafety is an AI-powered app that aims to tackle the issue using the FDA’s open-source database.
The Food and Drug Administration (FDA) has reported a total of 29 medical device recalls so far this year, of which seven were reported in July.
Most notably, software-related recalls are on the rise due to the increasing sophistication of medical device technology.
Recalls occur when a medical device is defective, when it could be a risk to health, or when it is both defective and a risk to health, and its consequences can be severe.
President and CEO of Soom Charlie Kim said that the idea for the app was prompted by his own experience with a medical device recall that impacted his youngest daughter, Isabella.
He shared with NS Medical Devices that she was born with a health condition requiring a medical device to help her breathe, but a subsequent recall due to a manufacturing defect was not communicated to him or the doctor treating his daughter.
Mr Kim said: “I only learned of the recall after the device failed, threatening the life of my daughter.
“As a patient advocate, I became acutely aware that my family’s situation was not unique — incomplete information in the medical device supply chain made it difficult to inform caregivers of recalls, and there was no public online database where they could seek out this information on their own.
“So, in 2015, when the FDA launched its openFDA databases — complete with a wealth of medical device product data like expiration dates and recall information — I recognised this could help resolve the information gaps.
“Using mobile scan and cloud-based technology, Soom could connect this data with individual medical devices, giving users across the healthcare value chain complete, accurate product data.”
How the SoomSafety app works
Boston-based start-up Soom, claims to have composed the first mobile app that bridges the information gap between the FDA, manufacturers and medical device users by harnessing the power of big data to advance the quality and safety of healthcare.
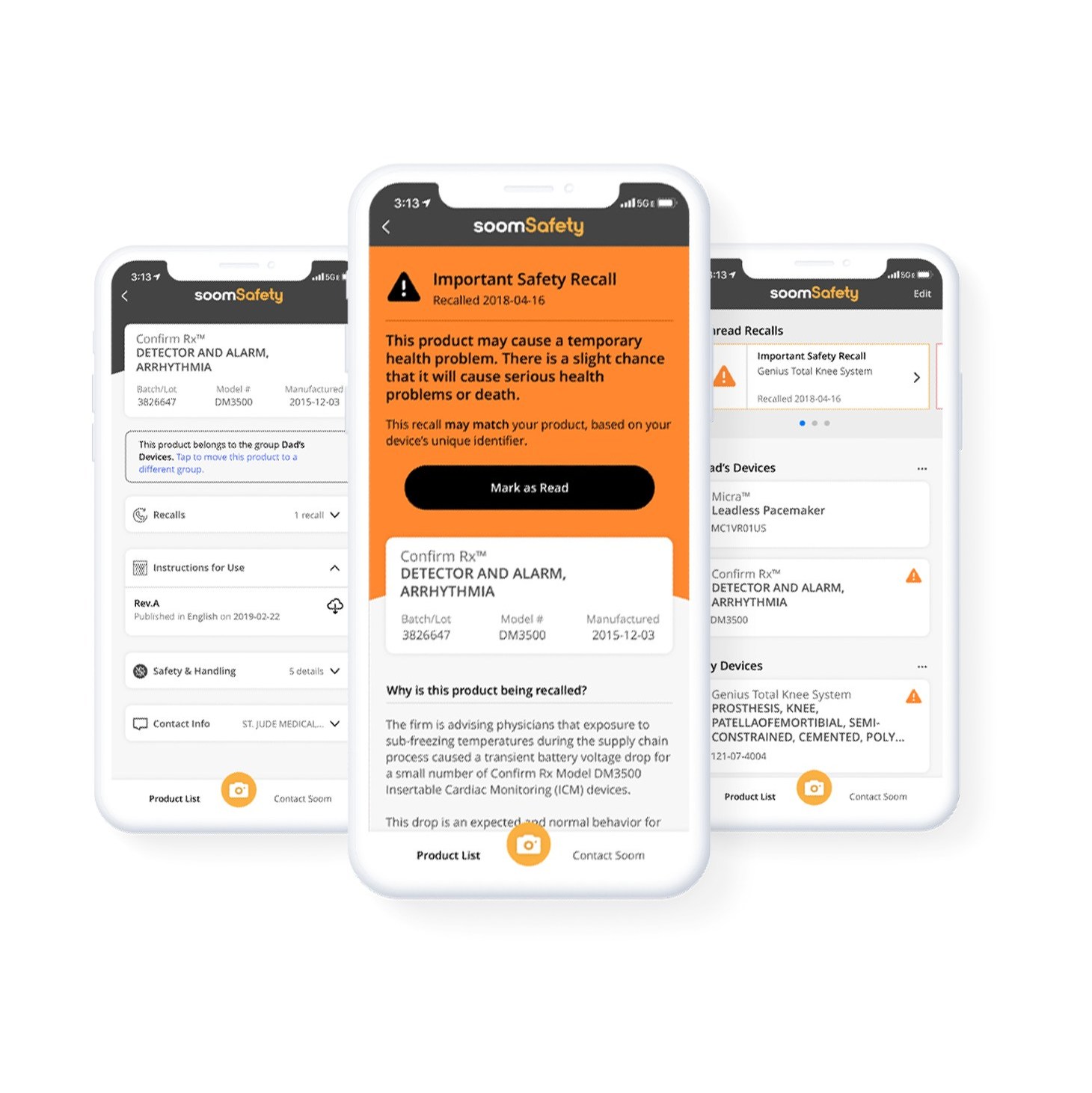
Once downloaded, the app allows patients, nurses and caregivers to simply scan the bar code of a medical device, such as an insulin pump, nebuliser or apnea monitor to bring up all safety and recall information about the product.
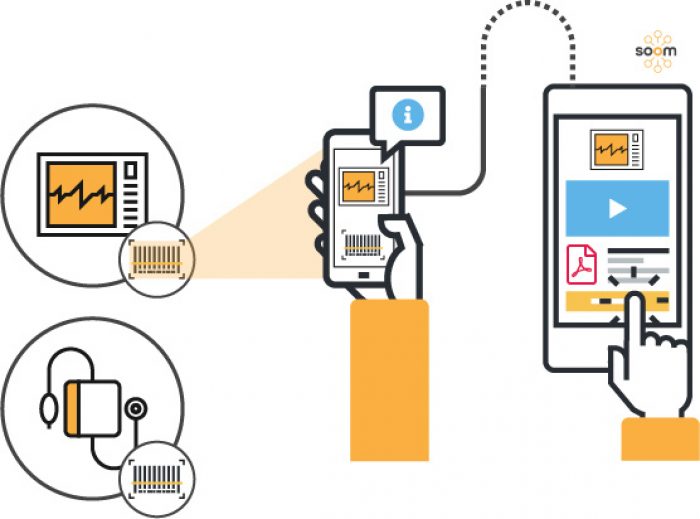
The app can be updated in real time, and also identifies and stores implanted medical devices like artificial joints, pacemakers and heart valves by scanning the barcode on a patient’s medical device identification card.
Both patients and providers have continued to show interest in the safety of medical devices — even when the FDA decides not to recall them.
Outside of fatalities, some of the most serious events reported were related to breast implant injuries, caused by leaks, deflation or migration.
More than half a million unique reports related to breast implants have been made, with 6,600 in 2019 alone, and the most common issue being ruptured implants.
This was the case earlier this year with women who had implants to either enlarge their breasts cosmetically or rebuild them after a mastectomy.
The app uses openFDA, an open-source database that enables developers to use FDA data in applications, enabling the data accessibility is to educate people and save lives.
“Many patients are never informed of these recalls due to incomplete information in the medical device supply chain,” Kim said.
“The launch of the FDA’s openFDA databases provided a wealth of product data, such as expiration dates and recall information.
“What was missing was a simple solution for connecting this data to individual medical devices
“Everyone should be able to answer the critical question ‘Is this medical device safe to use?’ And the answer needs to be as easy to find as one would experience with this app.
“Our technology makes it possible to connect previously soiled medical device data, giving patients — and their caregivers — more proactive control over their health and safety.”
What the FDA is doing to improve the safety of medical devices

In 2017, the FDA implemented a Voluntary Malfunction Summary Reporting (VMSR) programme, which enables it to efficiently detect potential safety signals and free up resources to better focus on addressing the highest risks, such as deaths and serious injuries, associated with medical devices.
The regulator will use new funding to make the Manufacturer and User Facility Device Experience database more user-friendly over the next few years, making medical device reporting data easier to find.
It also plans to begin designing active surveillance capabilities for its National Evaluation System for Health Technology, which has been in development since 2012 to systematically use real-world data to identify and address safety concerns around devices on the market.
FDA director Dr Jeffrey Shuren said: “Adverse events that result in a death or serious injury cannot be reported under the VMSR Program.
“Such events must still be reported to FDA within 30 days — or within five days if a problem with a device is particularly egregious.
“Active medical device surveillance will better protect patients by continuously using analytical software algorithms to evaluate large data sets on device performance, and patient safety associated with device use in routine clinical practice.”
Kim added: “The regulations currently enforce (US UDI, EU MDR) rules around post-market surveillance, which address safety and recall.
“The FDA publishes post-market information about device types in general so stakeholders can monitor risk.
“We know that for safety, identifying the exact item being recalled is relevant and important to the patient using the device.
“While all of this information is accessible via openFDA, Soom filters it to be more digestible and relevant to the end-user.”






