Digital biomarkers could play a key role in identifying patients most at risk from coronavirus as part of wider government strategies to contain the deadly disease, believes a healthcare expert.
Physiological and behaviour data collected from health-related technology like wearable devices and mobile apps enables doctors to continuously monitor Covid-19 symptoms.
Smart thermometers, for example, could alert users and linked-up healthcare professionals about a rise in body temperature at an early stage before a fever – one of the key coronavirus symptoms – develops and prevent further transmission.
And as remote patient monitoring (RPM) accelerates, Roxanne Balfe, senior digital healthcare analyst at GlobalData, says this could help governments keep track of the disease’s progression and any new outbreaks.
“The increase of digital tools such as the rise in popularity of health-related mobile apps and wearable devices has produced a novel set of biomarkers in large, diverse and complex data,” she said.
“During the on-going Covid-19 pandemic, digital biomarkers are emerging as important predictive tools to collect objective, quantifiable, physiological and behavioural data through remote, digital devices.
“This data can be used to explain, influence and predict health-related results, which could be crucial in providing a proactive and personalised approach to the management of the Covid-19 pandemic.”
Examples of how digital biomarkers could help battle against coronavirus
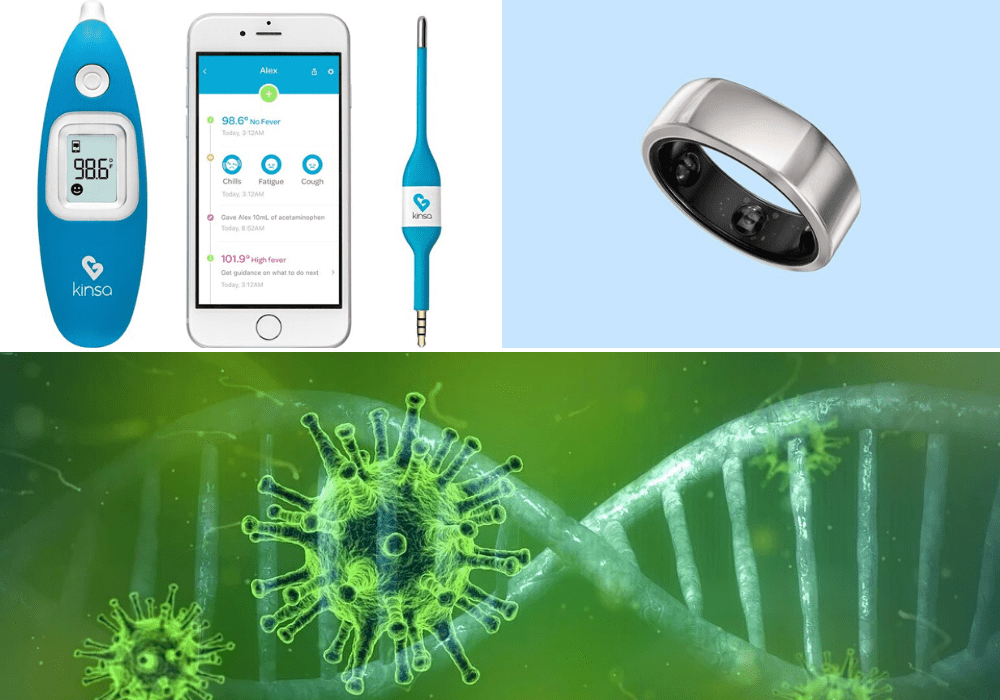
Kinsa Health
San Francisco healthtech start-up Kinsa Health’s smart thermometer and paired phone app have used to track flu outbreaks across the US over the past two years.
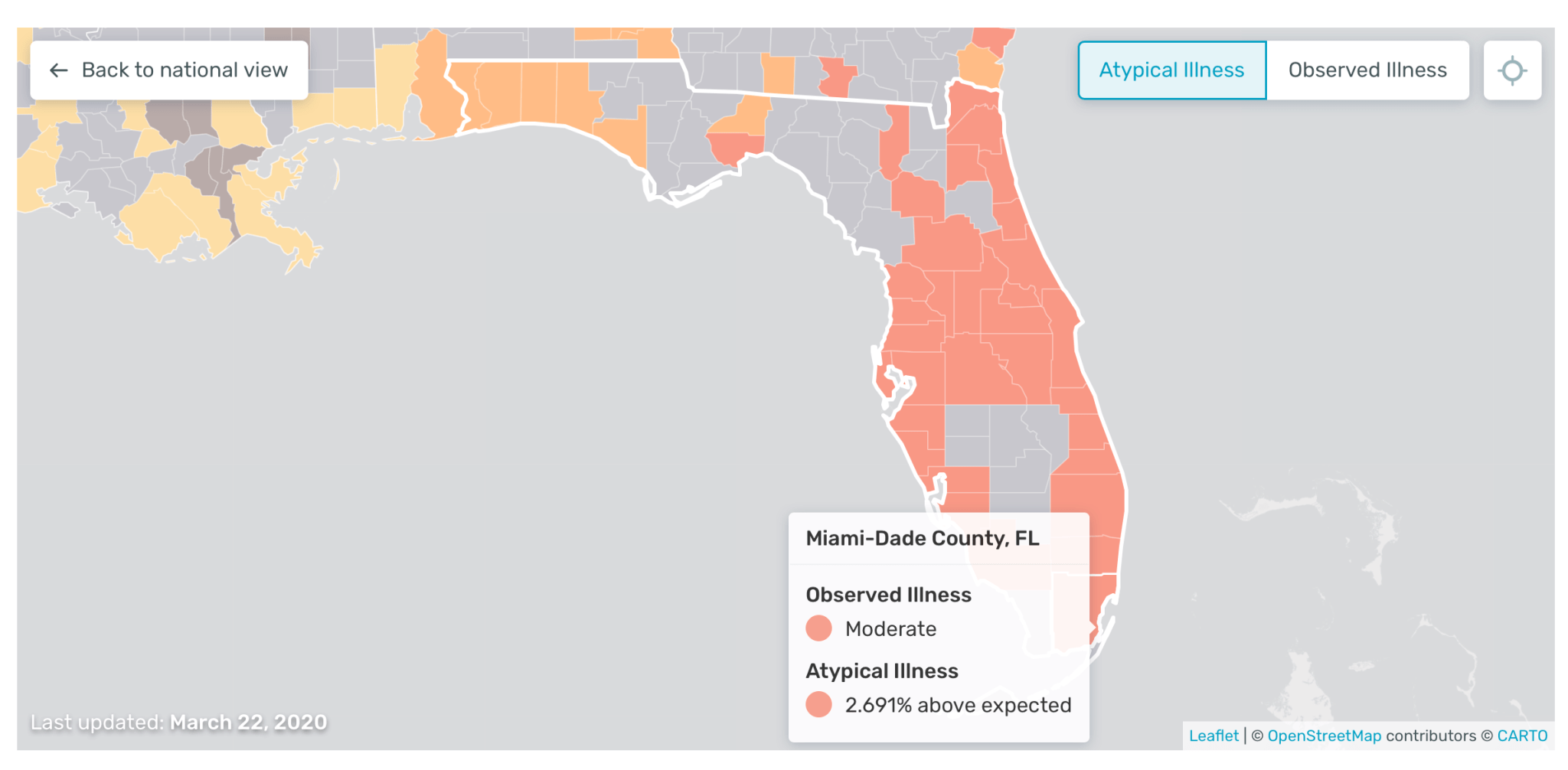
It features an interactive “US health weather map” that provides granular, real-time data. The company is now leveraging the technology as an early warning system to help healthcare organisations predict outbreaks of Covid-19 earlier.
This could enable a more rapid and efficient response, dampening the pressure on local healthcare systems.
Kinsa Health, which had a million thermometers on the market in February, reported receiving orders for up to 10,000 devices a day in March.
This has also led to the company increasing its data set, while it also has a feature on the map called “atypical illness levels” that could help shed more light on the transmission of Covid-19 across the US and the impact of strategies such as social distancing.
Founder and CEO Inder Singh told TechCrunch last month: “We’re taking our real-time illness signal, and we’re subtracting out the expectation.
“So what you’re left with is atypical illness. In other words, a cluster of fevers that you would not expect from normal cold and flu time.
So, presumably, that is Covid-19; I cannot definitively say it’s Covid-19, but what I can say is that it’s an unusual outbreak. It could be an anomalous flu, a strain that’s totally unexpected. It could be something else, but at least a portion of that is almost certainly going to be Covid-19.”
Oura Ring
Finnish tech company Oura, meanwhile, has teamed up with University of California, San Francisco (UCSF), to use its multi-sensor “smart ring” to identify digital biomarkers indicating the onset of Covid-19 among key healthcare workers.

The UCSF TemPredict study, as it’s known, has begun with 2,000 frontline workers who will each wear a smart ring, which is worn on the finger and detects key signals from the body with the usual purpose of tracking sleep and activity.
The coronavirus project will instead take data on body temperature, respiratory rate and heart rate on the accompanying Oura app, allowing health workers to monitor changes in these measures that could indicate early signs of infection so they can take the necessary action to prevent it spreading further.
Oura hopes to extend the study to a wider base of more than 150,000 Oura Ring users who will be able to opt-in to the study, generating a larger and more diverse data pool.
Medopad
A third example of companies repurposing existing technology for the Covid-19 pandemic is UK healthtech start-up Medopad, which has provided its remote patient monitoring software to NHS trusts and global healthcare providers.
The company has worked with clinicians from Imperial College London, Guy’s and St Thomas’ NHS Foundation Trust, and John Hopkins University to develop a specific Covid-19 digital biomarker based on its modular RPM platform – which allows hospitals to pool patient data into a single dashboard so it can be served to doctors’ mobile devices in real time.
Patients outside hospitals will be able to opt-in to securely share personal health data, including vital signs such as temperature, respiration rate and heart rate, using their smartphone.
It means doctors can monitor ill and at-risk patients away from clinical settings, give care to chronically ill patients and provide insights to healthcare systems so they can prioritise resources and respond more effectively.
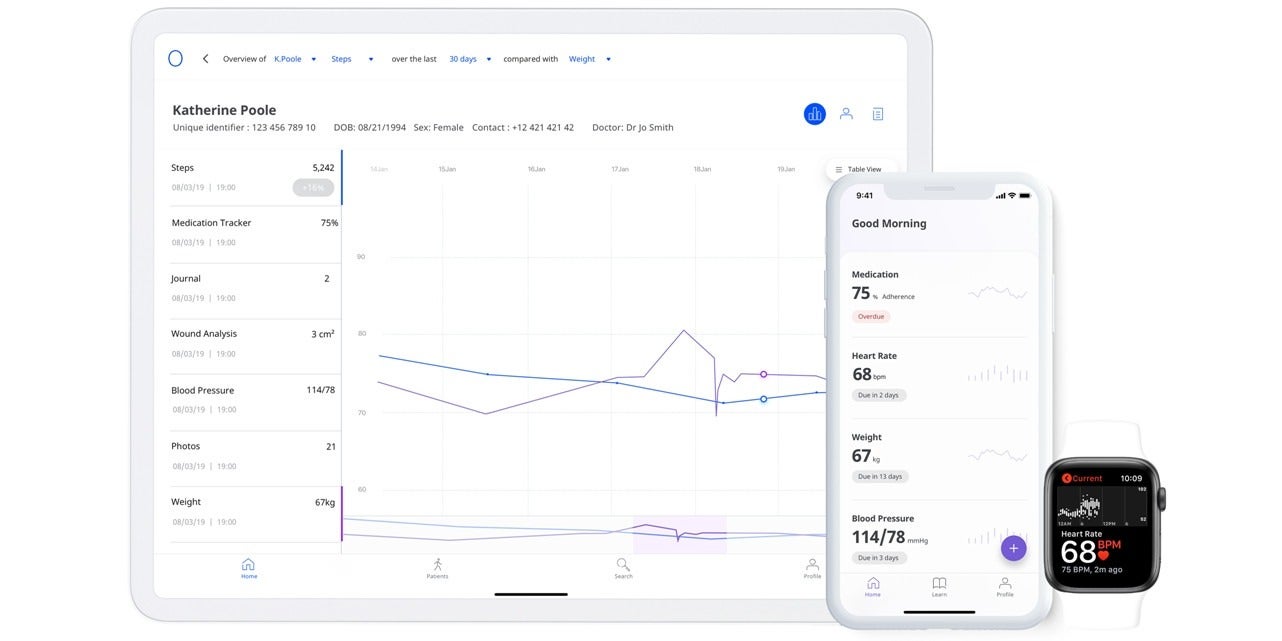
Felix Vaal, senior project manager for digital innovation at Chelsea and Westminster Hospital NHS Foundation Trust, which has previously used the RPM software for diseases including heart failure, said: “During the Covid-19 crisis, Medopad’s solution can allow hospitals to continuously monitor and engage with their patients without the need to bring them into the healthcare system where there is an elevated risk of them contracting Covid-19.”
Flexible regulatory approach needed to enable growth of digital biomarkers in battling coronavirus
While RPM technologies aren’t new, their popularity has certainly surged in recent years attributed partly to declining cost, software powered by artificial intelligence and advanced technical capabilities.
But the use of digital biomarkers to support preventive and predictive medicine is a far more novel concept that requires a collaborative approach to overcome issues around data quality, security and validation.
GlobalData analyst Balfe said: “The nature of digital biomarkers, reliant on consumer devices such as apps, mobile phones and wearables, highlights the need for alternative validation strategies for digital biomarkers.
“Traditional clinical trials that rely on double-blinded, randomized controlled studies as the gold standard are not flexible or responsive enough for emerging healthtech.
“Therefore, flexible regulatory pathways are vital to ensure digital biomarkers can fulfil their full potential, with multiple research organizations and companies collaborating to address these questions amidst the Covid-19 pandemic.
“The common goal among both healthtech companies and collaborators is to leverage digital technologies to identify digital biomarkers for Covid-19 such as temperature and heart rate, which will help to curb the spread of the virus.
“GlobalData believes that collection and analysis of these routinely-measured data points will be used to detect outbreaks much earlier than standard methods and prevent transmission more rapidly.
“This is key to mitigating the spread and impact of the virus.”






