
The unprecedented demand for Covid-19 testing kits seen last year resulted in many of the world’s biggest diagnostics companies being thrust into the limelight.
The likes of Roche, Abbott and Thermo Fisher Scientific were among numerous global firms that raced to develop fast, accurate methods for detecting patients infected with the SARS-CoV-2 virus and, just as importantly, how quickly it was spreading among populations.
It should therefore come as no surprise that many analysts are anticipating the market for in vitro diagnostic (IVD) products will significantly increase in value, at a CAGR (compound annual growth rate) of roughly 4.5%, over the next few years.
With the biggest companies in the diagnostics sector likely to both contribute to and benefit from this expected growth, we take a look at who the major players in the industry are right now.
The world’s biggest diagnostics companies in 2021
1. Abbott Laboratories
Illinois-based firm Abbott is currently one of the biggest medical device manufacturers in the world, and operates across a wide range of sub sectors including cardiovascular and diabetes care, pharmaceuticals, neuromodulation and diagnostics.
Within molecular diagnostics, its current portfolio includes a range of lab-based tests used to detect genetic disorders, infectious diseases and cancers.
Through its portable, hand-held blood analysers, Abbott is also looking to meet the growing need for point-of-care testing kits capable of producing fast, accurate and reliable results.
And, throughout the global pandemic, the company has gained regulatory approvals for multiple SARS-CoV-2 tests, with its BinaxNOW COVID-19 Ag Card Home Test becoming the first at-home, virtually guided coronavirus diagnostic to gain an FDA EUA (emergency use authorisation) on 16 December 2020.
Abbott currently has more than 109,000 employees across the world and reported a total revenue of $34.6bn for 2020.
2. bioMérieux
French biotech company bioMérieux specialises in diagnostic solutions – and provides IVD devices, reagents, software and services used by laboratories across more than 160 countries globally.
Its main focus is the detection of infectious diseases including HIV, sepsis, gastrointestinal infections, and respiratory diseases like pneumonia and the novel coronavirus.

In the summer of 2020, bioMérieux gained a CE marking and an EUA for its VIDAS SARS-CoV-2 test – which is an immunoassay used to detect IgM and IgG antibodies, and diagnose individuals previously infected with the virus.
The company reported total revenues of €3.12bn ($3.6bn) for 2020, and currently employs about 12,800 people globally.
3. Bio-Rad Laboratories
Bio-Rad is an American developer and manufacturer of products for the life sciences research and clinical diagnostics markets.
Its work in the latter sector ranges from autoimmune and diabetes testing, and newborn screening, to diagnosing bacterial or viral infections.
Bio-Rad’s Reliance SARS-CoV-2 RT-PCR Test – a molecular diagnostic for individuals suspected by their healthcare provider of having Covid-19 – gained an FDA EUA on 15 January 2021.
The California-based firm reported revenues of $2.5bn for 2020, and employs more than 8,000 people across 35 countries.
4. Danaher Corporation
Healthcare giant Danaher’s work takes place under the three main sub sectors of environmental and applied solutions, life sciences – including biopharmaceuticals and clinical research – and diagnostics.
Much of its business in the diagnostic industry is carried out via subsidiaries like Beckman Coulter – a company it acquired in 2011 for about $5.8bn.
Beckman Coulter develops numerous products for diagnosing and detecting sepsis, prostate cancer, UTI (urinary tract infection), cardiovascular disease, anaemia, and several other health conditions.
Beckman Coulter’s serological IgG and IgM antibody tests for SARS-CoV-2, which are designed for use on any of the company’s Access immunoassay systems, also gained EUAs in June and October respectively of last year.
In 2020, Danaher’s reported total revenue stood at $22.3bn, and the company currently employs more than 70,000 people globally.
5. F. Hoffmann-La Roche
The two main divisions within Swiss multinational healthcare firm F. Hoffman-La Roche – often shortened to Roche – are pharmaceuticals and diagnostics.
Its in vitro diagnostics solutions cover a range of areas including clinical chemistry, immunoassays, molecular and digital diagnostics, point-of-care testing, patient self-testing and next-generation sequencing – much of which is supported by its industry-leading cobas platform.

Roche also became one of the first companies to receive authorisation to sell a Covid-19 diagnostic in the US and Europe when its cobas SARS-CoV-2 RT-PCR test received FDA approval and CE certification in March 2020.
The Basel-based firm posted total revenues of $64.66bn in 2020 and employs more than 100,000 people across the world.
6. Qiagen
European-American biotech firm Qiagen has headquarters in the Netherlands, Germany and the US, and provides a range of technologies used in molecular diagnostics and clinical research.
Within in vitro diagnostics, it offers testing solutions for infectious diseases – most notably TB, HIV, hepatitis, herpes and cytomegalovirus (CMV) – as well as oncology, and immune response modulation.
On 30 March 2020, Qiagen’s QIAstat-Dx Respiratory SARS-CoV-2 Panel, which is an RT-PCR test for detecting and differentiating nucleic acids from multiple respiratory viral and bacterial organisms, received an FDA EUA that allowed it to be used for diagnosing Covid-19.
Qiagen recorded a total revenue of $1.87bn in 2020 and employs 5,600 people across 27 locations worldwide.
7. Quest Diagnostics
New Jersey-based company Quest Diagnostics offers a broad range of products and services for patients, physicians and companies alike.
Within diagnostics – which comprises the vast majority of its business – it produces everything from routine blood tests to complex, gene-based or molecular testing, as well as clinical trials testing and healthcare IT.
Quest Diagnostics’ claimed areas of expertise include oncology, cardiovascular diseases, infectious diseases, and neurology.
The range of IVD products Quest Diagnostics has developed to tackle the spread of Covid-19 includes a multi-analyte, home collection RT-PCR testing kit for SARS-CoV-2 and influenza, which gained an EUA in December 2020.
In 2020, the company reported total revenue of $9.44bn, and it currently employs about 48,000 people.
8. Siemens Healthineers
Siemens Healthineers is the healthcare arm of global manufacturing heavyweight Siemens.
Alongside its work in digital health, electronics and medical imaging, the German firm makes numerous products for use in laboratory diagnostics and point-of-care testing.
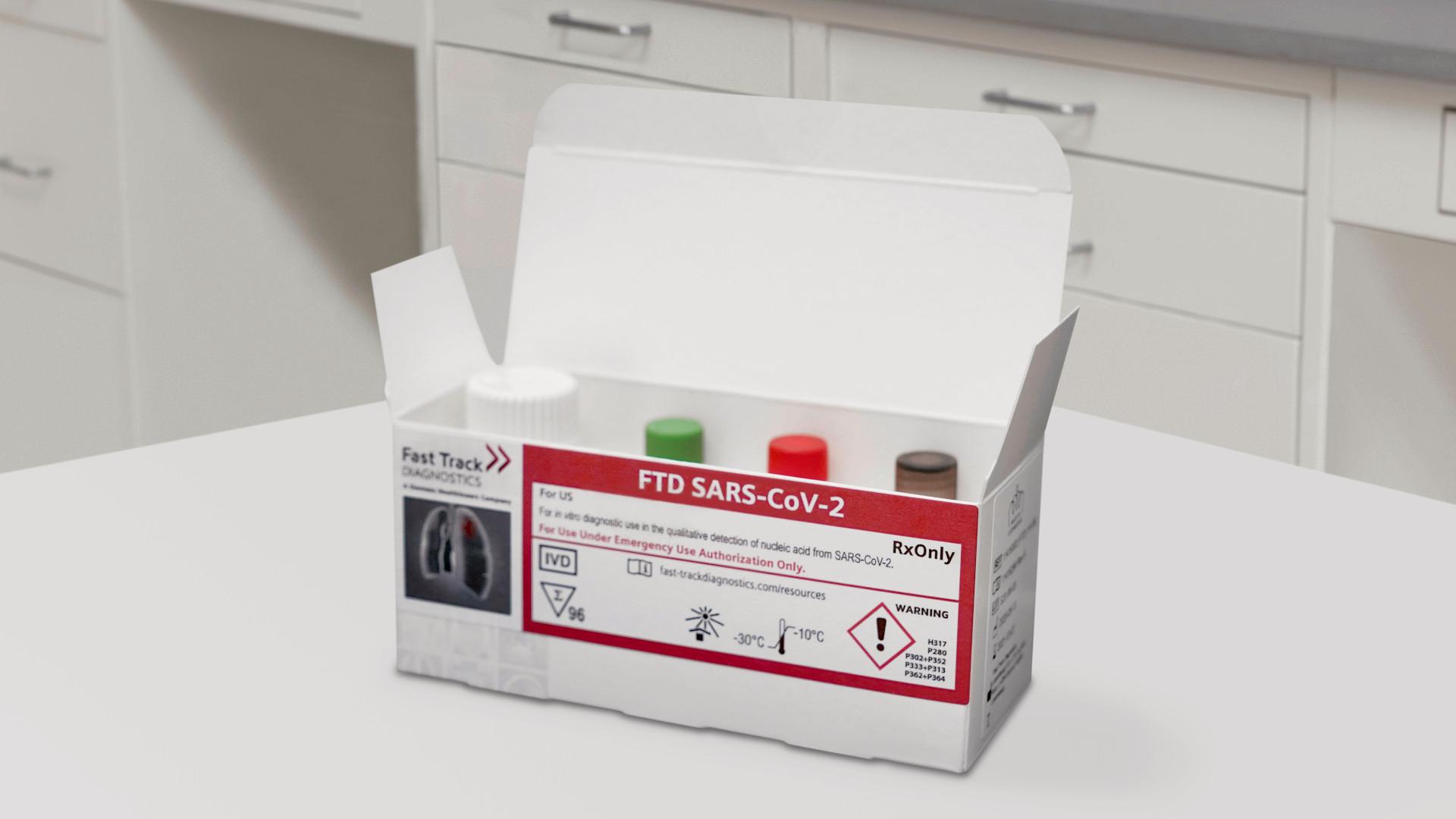
Through one of its many subsidiary companies in the IVD sphere, Fast Track Diagnostics, Siemens Healthineers has gained a CE mark and an FDA EUA for its FTD SARS-CoV-2 assay test – doing so in May 2020.
Siemens Healthineers’ total revenue in 2020 stood at €14.5bn ($17.7bn), and it currently has about 54,000 employees working across more than 70 countries.
9. Sysmex Corporation
Japanese firm Sysmex is thought to be the leading APAC (Asia-Pacific)-based company in the global diagnostics sector.
Although it specialises in haematology diagnostics – more commonly known as blood testing – Sysmex’s product portfolio also contains solutions used in molecular diagnostics, urinalysis, digital imaging, oncology and flow cytometry.
The company gained first marketing approval in Japan for its 2019-nCoV Fluorescence Detection Real-Time RT-PCR Kit on 27 March 2020 – and began delivering it to medical institutions across the country in that same month.
Sysmex reported total revenues of $2.87bn for the fiscal year that ended in March 2021, and employs more than 9,200 people globally.
10. Thermo Fisher Scientific
American firm Thermo Fisher Scientific works across much of the life sciences and healthcare sectors, and produces a vast range of reagents, instruments and technologies that are used within diagnostics as well.
The key areas it focuses on here are allergies, autoimmune diseases, diabetes, blood testing, oncology and transplant diagnostics.
Like Roche, Thermo Fisher Scientific became one of the first companies to gain FDA approval for a novel coronavirus diagnostic when its TaqPath COVID-19 Combo Kit – an RT-PCR, home collection test – was granted an EUA in March 2020.
In 2020, Thermo Fisher Scientific reported revenues of $32.2bn. The company also currently employs about 80,000 people worldwide.






