Absorbable medical device development has been hindered by a lack of materials that can exhibit a wide range of tensile moduli similar to the various tissues where devices are implanted. Poly-Med, Inc. has opened the door to solving these challenges through its material portfolio and vertically integrated manufacturing processes.
Bioabsorbable medical devices offer significant advantages compared to their biostable counterparts, due to their ability to degrade over time by providing support while tissue reinforcement is needed and degrading into biologically safe by-products when it is not.
PIC 1 CAP: Poly-Med’s bioabsorbable polymers are designed with segmented block copolymer structures, increasing toughness and elasticity.
Although the idea of utilising synthetic degradable materials in medical devices has been used in the clinic for 50 years with the launch of the Dexon suture in 1972 by Davis & Geck, absorbable medical devices have yet to capture large market shares. In part, this is due to the lack of materials and processes suitable to create implants exhibiting mechanical properties matched to the target tissue and indication requirements. Additionally, several medical devices have been recalled due to biocompatibility issues with select long-lasting absorbable materials stemming from poor material selection.
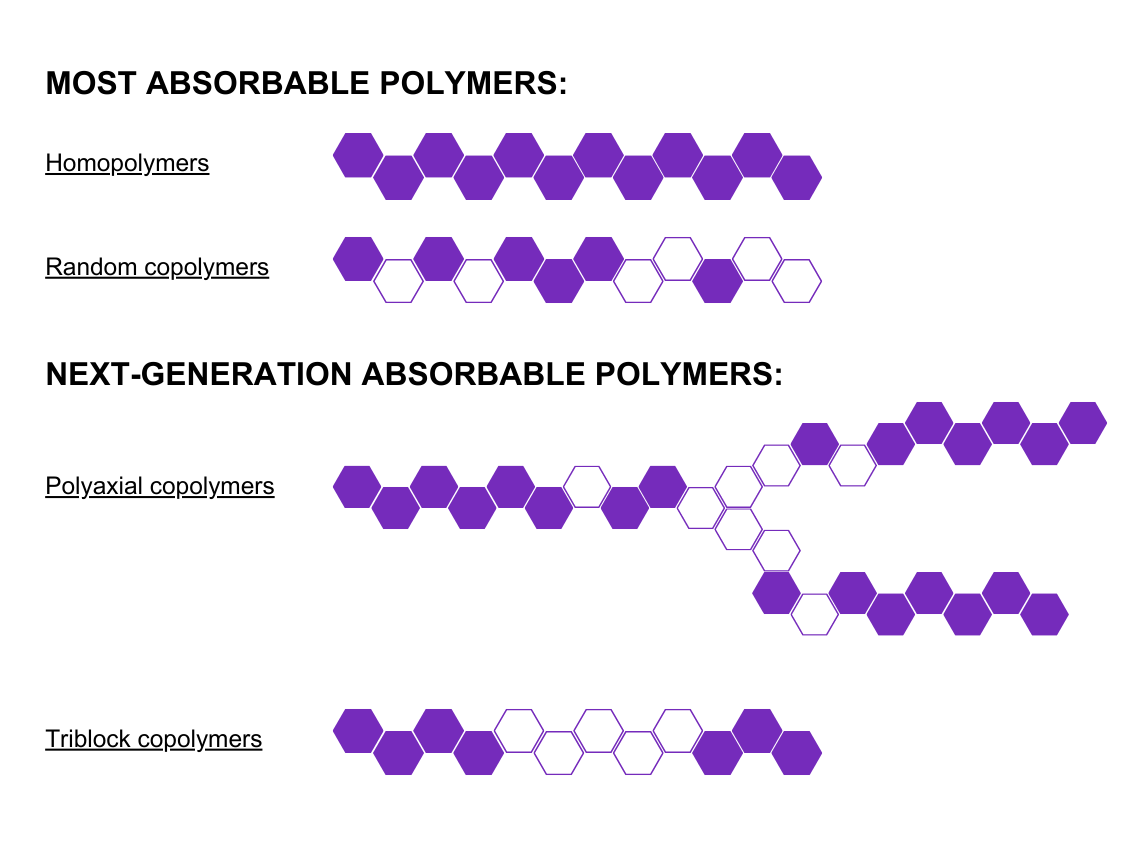
Poly-Med, Inc. offers a solution to these challenges by manufacturing next-generation absorbable block copolymers that exhibit increased toughness and biocompatibility compared with traditional linear homopolymers and random copolymers. Poly-Med, Inc. can achieve preferred mechanical and biological properties by controlling chemical composition and polymer structure.
Increased speed to market with a vertically integrated partner
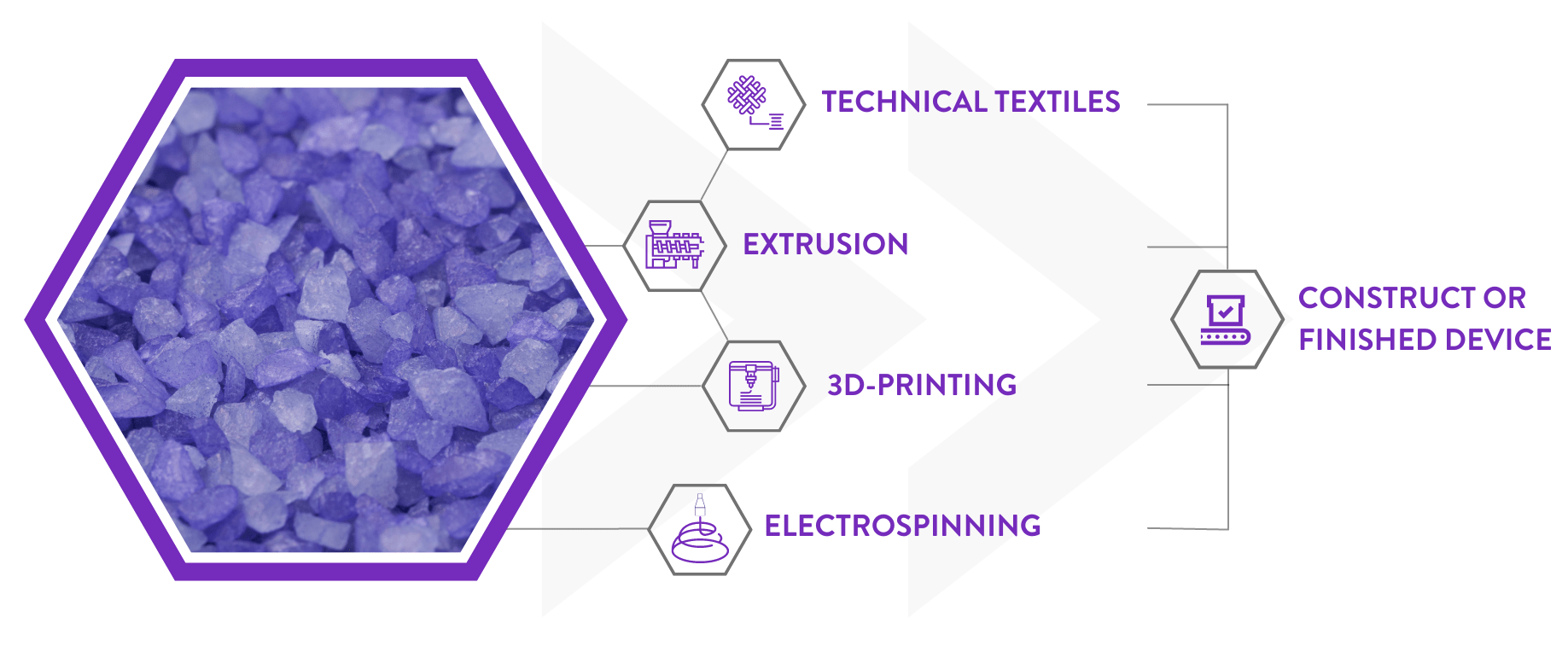
Although the materials from Poly-Med, Inc. offer design advantages over traditional absorbable polymers, these materials can also be more challenging to process into a desired form factor. To circumvent additional challenges with processing these nextgeneration absorbable polymers, Poly-Med, Inc. can act as a vertically integrated partner to convert these materials into semi-finished and finished medical components, or into packaged medical devices ready for sterilisation.
This vertically integrated supplier network is most advantageous when developing implantable devices such as surgical meshes or sutures, where the team from Poly-Med, Inc. can synthesise the selected polymer, extrude the polymer into a monofilament fibre or multifilament yarn, and then knit or braid the extruded materials into a desired construct.
PIC 2 CAP: Poly-Med is a vertically integrated partner, from bioabsorbable polymer to finished implantable medical device.
Over the past five years, Poly-Med, Inc. has been able to expand its vertically integrated service offerings into advanced manufacturing approaches, such as electrospinning to create nanofibrous textiles and 3D printing to create porous structural elements. Poly-Med, Inc. currently offers GMP-grade filament for fused deposition modelling (FDM) 3D printing that can enable the creation of complex lattice geometries, and offers significant advantages over solid, monolithic designs typical of injection molded parts. More recently, PolyMed, Inc. has launched its Photoset resin, a biocompatible and absorbable photopolymer that can be crosslinked into 3D-printed parts via commercially available stereolithography (SLA) and digital light processing (DLP) 3D printers. With the combination of an advanced bioresorbable material portfolio and wide array of conversion processes capable of creating a variety of structures and resultant properties, Poly-Med, Inc. can iterate faster to increase an implantable medical device’s speed to market, to ultimately support product development to the benefit of patients who require temporary tissue reinforcement.






