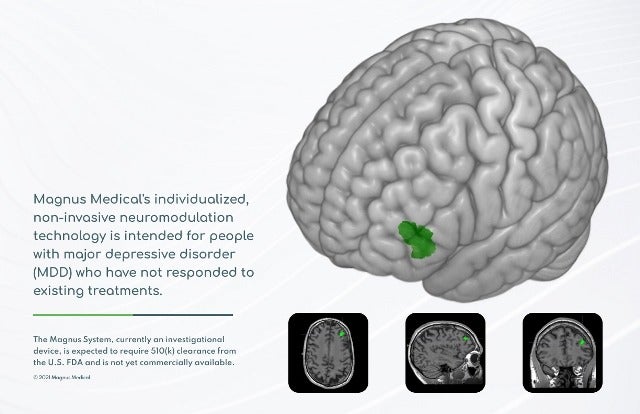
Magnus Medical has secured a breakthrough device designation from the US Food and Drug Administration (FDA) for its neurostimulation technology to treat major depressive disorder (MDD).
The individualised, rapid-acting and non-invasive neurostimulation technology has been designed for the treatment of MDD in people who have not improved sufficiently from antidepressant medication or other treatments.
Magnus Medical has also secured $25m under the Series A financing round co-led by JAZZ Venture Partners and Red Tree Venture Capital.
The Magnus system is developed using SAINT technology, which is exclusively licenced by Magnus Medical from Stanford University for commercialisation.
Magnus Medical co-founder and CEO Dr Brett Wingeier said: “We are thrilled that the FDA has recognized the novelty and potential of our therapeutic approach for major depression.
“Treatment-resistant depression causes enormous human suffering, and it’s imperative to find a truly effective solution. With individualized, accelerated treatment we will finally be able to offer people with treatment-resistant depression the ability to manage their symptoms acutely, and eventually stay well for a lifetime.”
The investigational double-blinded randomised controlled trial (RCT), which assessed SAINT technology, showed that it holds the potential to deliver rapid and better treatment for severe, refractory depression in a broader clinical setting.
As part of the trials, 14 study participants secured active treatment and another 15 received sham (placebo) treatment.
The clinical study results demonstrated that 79% of people in the active treatment arm entered remission compared to only 13% people entering into emission in the sham treatment arm.
In an earlier pilot study assessing SAINT, 19 of 21 study participants (90%) entered remission, stated the company.






