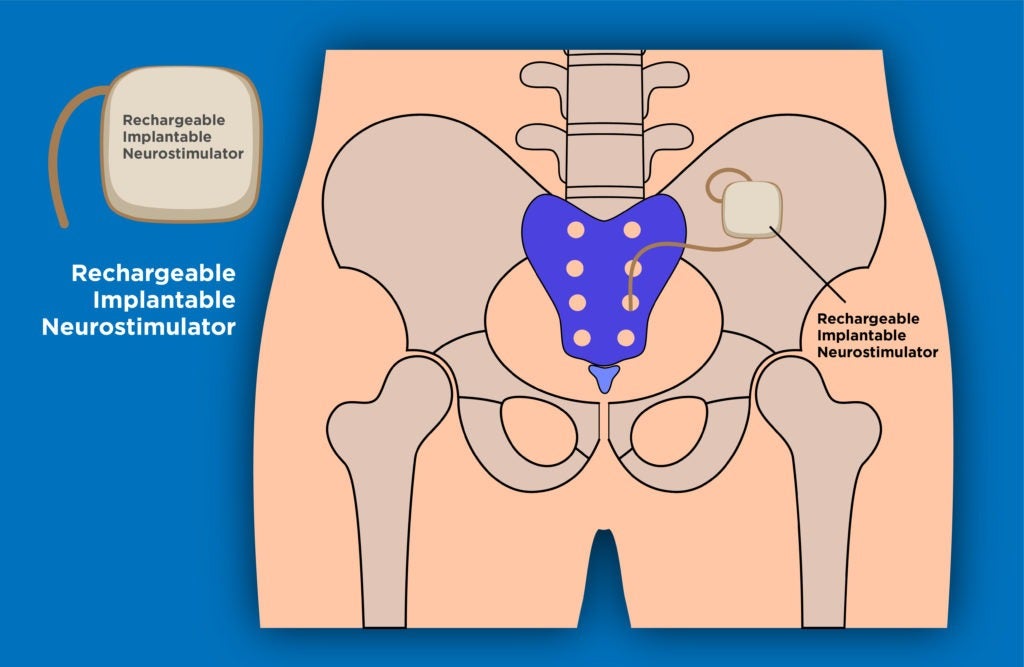
Aptar CSP Technologies has received the US Food and Drug Administration (FDA) approval for its Activ-Film technology for use with Implantable Neurostimulator (INS).
Activ-Film technology is intended for use within a recently launched Rechargeable Implantable Neurostimulator that treats urinary and bowel dysfunction.
Aptar CSP said that its Activ-Film material is designed to integrate into the medical device to achieve humidity control, improved accuracy of readings, and extend the use life.
Aptar CSP Technologies commercial operations vice president Badre Hammond said: “This is another example of our consistent commitment to helping customers bring advanced solutions to patients and improve user experiences and outcomes.
“We are dedicated to continued material science innovation that facilitates the development and commercialisation of next-generation implantable medical devices.”
The company said that the Activ-Film material is a configuration of its patented 3-Phase Activ-Polymer technology platform.
It manages the device’s internal atmosphere by adsorbing moisture, which could otherwise accumulate and affect the implant’s stability and performance.
Controlling the humidity within the device will protect the battery and facilitates little to no degradation of the device during its use life, said the company.
In addition to protecting a wide range of implantable medical devices, the highly-engineered technology has several applications in oral solid dose drugs, drug delivery systems and probiotics.
Aptar CSP is a part of AptarGroup, engaged in the design and manufacturing of drug delivery, consumer product dispensing and active material science solutions.
Using material science technology, it delivers personalised and integrated active solutions that adsorb moisture, scavenge oxygen, odour, or VOCs, emit aromas, or reduce pathogens.
Earlier this year, the company’s Activ-Film technology was selected to protect a new SARS Rapid Antigen test Covid-19 that recently received FDA Emergency Use Authorisation (EUA).






