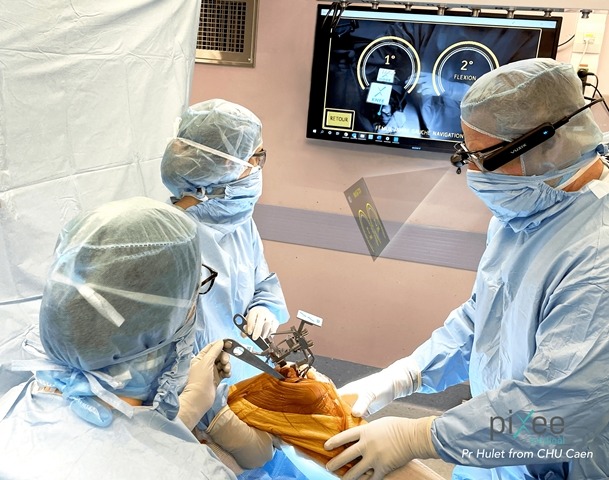
Pixee Medical has secured 510(k) clearance from the US Food and Drug Administration for its Knee+ AR computer assisted orthopaedic solution.
Knee+ is a patented platform developed to help orthopaedic surgeons to conduct surgeries efficiently and rapidly by offering real-time positioning of instruments in their field of view.
According to the company, Knee+ platform needs minimal training since it does not change the overall technique for 90% of surgeons who use a conventional technique.
The patented platform consists of a software using unique computer vision and artificial intelligence algorithms.
The computer assisted orthopaedic solution runs on connected smartglasses, thereby helping to avoid bulky capital equipment or disposables.
Pixee Medical founder and CEO Sébastien Henry said: “The FDA’s clearance of Knee+ is an important step forward as the USA represents 50% of the worldwide market. We plan to quickly expand our platform to perform hip and shoulder replacements.
“In addition, our platform is designed to become the cornerstone of data acquisition and exchange during surgery as well as a plug-and-play hub for accessories like connected instruments, robotic arms and wireless tools.”
In June last year, the first Knee+ surgery was conducted in Lariboisière Hospital in Paris. The company started commercialisation of the product in Europe and Australia in January.
Henry said: “Today, Knee+ is well positioned to help ambulatory surgical centers face the significant backlog and increase in knee surgery. These centers need intuitive, effective, attractive and affordable solutions to meet their patients’ needs, now.”
In May last year, the company secured CE mark approval for Knee+ orthopaedic navigation system for total knee arthroplasty.
The navigation system that leverages M400 Smart Glasses developed by Vuzix, a supplier of Augmented Reality (AR) technology products.






