
Testing for Covid-19 is an evolving field of study, and dependent on a pivotal trial, two companies are looking to commercialise a breath test for detecting the virus to the degree it can be used in mass testing. Peter Littlejohns explores the device, its efficacy and the company’s ambitions.
As countries begin the rollout of the SARS-CoV-2 vaccine from Pfizer and BioNTech, experts continue to remind people that there’s still a need to control the spread of the virus that causes Covid-19.
One recent article from The New York Times hammered home the message that masking and social distancing will still be necessary because the vaccinated can still harbour and transmit the virus, despite not getting ill from it.
So far, the effort to test and trace the spread to prevent such transmission has evolved from time-consuming RT-PCR conducted in a lab to rapid lateral flow tests applied en masse in some cases – although the latter technology has some important drawbacks.
But one new product could deliver the speed and accuracy needed to make mass testing a reliable endeavour, and people need only breathe into it and wait three minutes.
Canary Health Technologies and SmartShape Design – two companies based in Cleveland, Ohio – collaborated to create ASU Detect CV19, an ultra-rapid, highly accurate breath test for the detection of Covid-19.
According to the companies, the hand-held digital test requires minimal training and can be performed at the point of care, without the need of a laboratory.
Device inventor and Canary Health Technologies’ CEO Raj Reddy says: “Our cutting-edge technology enables easy, rapid testing every few days in order to optimise safety in workplaces and other settings.
“Our unique strength will be our ability to detect Covid-19 in under three minutes before the onset of symptoms, which will be critical in reducing transmission and ultimately putting an end to this pandemic.”
Pivotal trial of Covid-19 breath test
A key hang up of diagnostic tests during the pandemic has been the sacrificing of accuracy for speed, largely due to the time it takes to amplify viral RNA in a sample and increase the chance of detection.
In the UK this was seen in the roll out of mass lateral flow testing, a method that has come under increased scrutiny from the scientific community for its potential to give false negatives in cases with low viral loads.
Canary and SmartShape believe the sensitivity and specificity of their Covid-19 breath test is high due to preliminary studies ran to demonstrate the detection of lung cancer using the same technology.
The results of that 2019 study have yet to be published, but with non-PCR tests coming under scrutiny for sensitivity ratings far below 90%, it can be readily assumed that the results were better than that if the two companies wish to replicate them for Covid-19.
The study is currently underway in Delhi, India, and will include a sample of 750 people, some that do, and some that do not, have the virus.
Organisers of the study, which is being conducted in collaboration with local firm DIVOC Laboratories, have said they expect results by the end of December.
Trials in the United States are also being planned and due to begin before the end of 2020, but if the Delhi trial is successful, Canary says it will seek to move quickly to apply for fast-track regulatory approval while continuing to trial the test in real-world settings such as airports, resorts and other high traffic areas.
How does the Covid-19 breath test work?
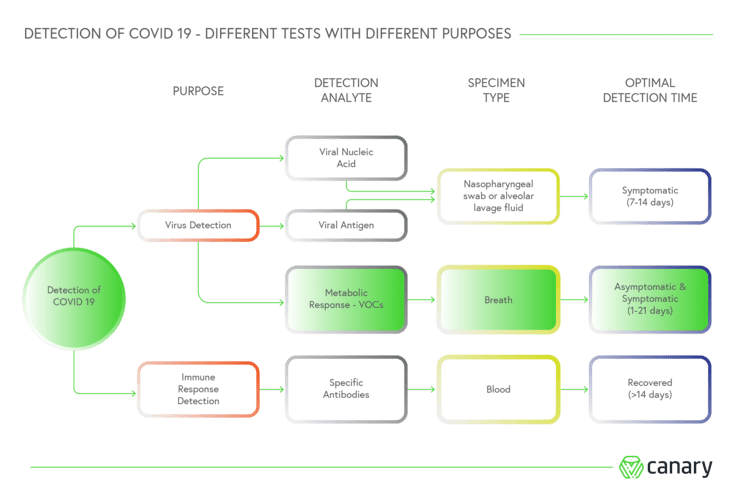
Using exhaled volatile organic compounds (VOCs) found in human breath as biomarkers of the virus, ASU Detect CV19 is designed to detect the SARS-CoV-2 virus whether people are exhibiting symptoms of Covid-19 or they’re asymptomatic.
While current tests measure the viral load in a person to determine if they are infected, Canary claims its breath test detects the metabolic response almost immediately after infection, and delivers results in less than three minutes.
Another aspect of the technology is its use of cloud-based artificial intelligence for pattern recognition as the analytical tool that delivers a result.
Disposable sensor cartridges are inserted into the base device, and when a person breathes normally into the mouthpiece, biomarkers are collected by the sensor and transformed into electronic signals.
The digitised breath data is relayed to Canary’s Sentinel Detect software running on a computer, where it is packaged and sent to the cloud for analysing – a process that takes an alleged milli-second to deliver a positive or negative result.
Will it be accurate?
VOC-based tests already exist for the detection of pulmonary diseases – those that affect the lungs – and the scientific literature available suggests a high degreed of variance in their sensitivity and specificity.
One recent academic review of VOC technologies applied for this subset of diseases noted that in the case of lung cancer – the focus as Canary’s early trials – the accuracy of a diagnosis could be above 90% for some sensors and far lower for others.
Canary is backing its proprietary sensor in its ambition to, as stated by Reddy, “optimise safety in workplaces” and for “reducing transmission”, as well as for mass screening and monitoring.
Until the results of the Delhi study are released it’s unclear whether the Covid-19 breath test has the potential to achieve those ambitions, but one thing is clear, if the sensitivity rating isn’t higher than in lateral flow tests, it’s likely to come under the same scrutiny.
Canary’s ambitions go beyond Covid-19, however, with 2021 trials planned to evaluate the use of its VOC-based testing device in detecting rheumatoid arthritis and tuberculosis, among other diseases.






