
When Lucira Health gained an EUA (emergency use authorisation) for its Covid-19 All-In-One Test Kit last month, it became the first company to receive US FDA approval for an at-home, self-testing diagnostic during the coronavirus pandemic.
The test – which was five years in the making, having been developed as a diagnostic for the flu initially – has catapulted a relatively small biotech firm with just 40 employees into the public eye.
And Lucira Health is certainly not standing still following this momentous seal of approval from the FDA – as it announced upon receiving the EUA, the company is already scaling up its manufacturing capabilities and will also seek additional indications for its self-testing kit in 2021.
We take a closer look at Lucira Health, the work it undertakes outside the context of the global coronavirus pandemic, and what the future may hold for this pioneering US firm.
History of Lucira Health
Lucira Health was co-founded in 2013 by current chief technology officer Dr Debkishore Mitra, and board member and entrepreneur John Waldeisen, under the name ‘Diassess’.
Based in Emeryville, California, biotech firm Diassess was created with the intention of producing inexpensive, disposable diagnostics capable of turning any smartphone into a portable, real-time health monitoring device.
And, while it was rebranded as Lucira Health in May 2019, the company’s broad mission of “reimaging infectious disease testing” and enabling “rapid, accessible, multiplexed analysis of bodily fluids anywhere in the world” has remained the same.

Today, Lucira Health has about 40 employees at its headquarters near the University of California, Berkeley – one of the biggest public research universities in the US – and has raised a total of $52m in funding since its inception seven years ago.
The company’s current CEO and president, Erik Engelson, was also previously the head of American healthcare firm Medina Medical – which developed a device for treating cerebral aneurysms and was acquired by US medical device giant Medtronic for $150m in 2015.
First approved at-home testing kit for Covid-19
On 17 November 2020, the Lucira Covid-19 All-In-One Test Kit received an EUA from the US Food and Drug Administration – meaning that, while it has not been fully approved by the regulator, it can be used in at-home testing for the novel coronavirus during the global pandemic.
Specifically, it can be obtained via prescription by American patients aged 14 or over who are suspected of having Covid-19 by their healthcare provider, although it can also be used in patients under the age of 13 if their sample is collected by a healthcare professional.
The Lucira test harnesses molecular testing – the gold standard in diagnosing infectious diseases – and works by identifying the genetic material associated with the SARS-CoV-2 virus in patient samples taken using a nasal swab.
This differs from antigen tests, which detect certain proteins found in the virus, and are broadly considered less reliable but achieve a higher throughput of results.
Lucira Health’s single-use, handheld device is designed to be user-friendly, and involves three simple steps – patients swab their nose, stir the swab into a sample vial, and then wait for the test’s display to indicate a positive or negative result.
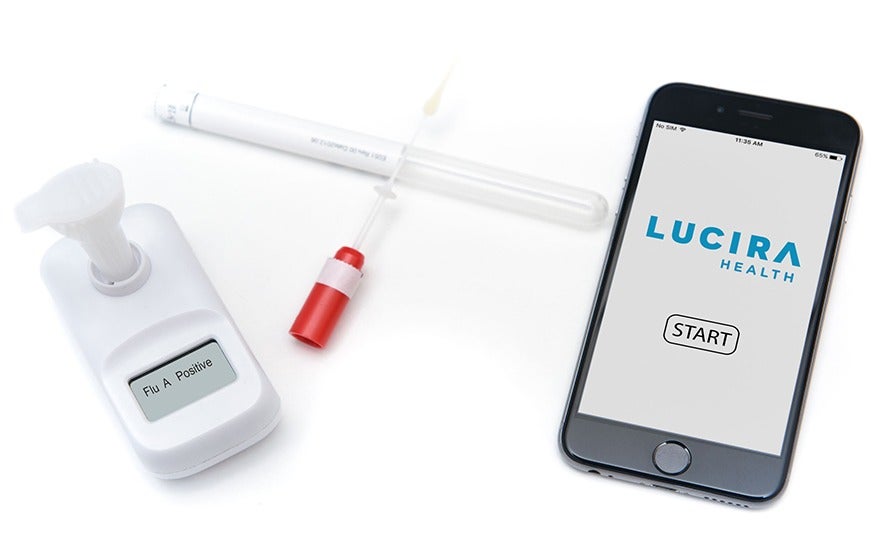
Lucira Health claims its test will produce either of these results within half an hour – although a positive result will often take as little as 11 minutes – and, in clinical trials, 100% of patients over the age of 13 were able to perform their own test in about two minutes.
In a separate Community Testing Study, the Lucira test was compared to an FDA-authorised, high sensitivity SARS-CoV-2 test, and achieved a 94% positive percent agreement (PPA) and a 98% negative percent agreement (NPA) – indicating it is more accurate in detecting the virus than many currently-approved Covid-19 antigen testing kits.
Upon the test being given an EUA, FDA commissioner Dr Stephen M Hahn said: “While Covid-19 diagnostic tests have been authorised for at-home collection, this is the first that can be fully self-administered and provide results at home.
“This new testing option is an important diagnostic advancement to address the pandemic and reduce the public burden of disease transmission.”
The future of Lucira Health
As of December 2020, the Lucira Covid-19 All-In-One Test Kit is expected to be available “in the near future” to patients served by two specific healthcare centres – Sutter Health in Northern California, and Cleveland Clinic Florida in Miami-Fort Lauderdale.
By early-spring 2021, however, the company expects its test to be available nationally through many more healthcare providers.
Lucira Health is currently working on scaling up its manufacturing capabilities in order to roll out as many of its at-home Covid-19 tests as possible.

But, as this process is ongoing, the Lucira test will initially be available on a limited basis in point-of-care (POC) settings, and through healthcare networks that prescribe the test for patients to use at home.
The company is anticipating its test will cost about $50 – which it says will help to make home testing for the virus more accessible in the US.
Lucira Health has also announced that, by the second quarter of 2021, it will try to amend its EUA – or file a new EUA – so patients who think they’re infected with Covid-19 can communicate with a medical professional online through a dedicated website to arrange a prescription and overnight delivery of the test kit.
While it’s clear Lucira Health will have its hands full rolling out this at-home testing kit across the US in the immediate future, it’s also likely that gaining approval for such a high-profile, breakthrough device could provide a strong platform for the company to expand its portfolio of point-of-care diagnostics in the long run as well.






