Quadrant Biosciences, a developer of epigenetic diagnostic tools, has secured the US Food and Drug Administration (FDA) Emergency Use Authorization (EUA) for its Clarifi Covid-19 Test Kit.
The Clarifi Covid-19 test is developed under partnership with SUNY Upstate Medical University, and leverages Quadrant’s expertise in RNA-based diagnostics.
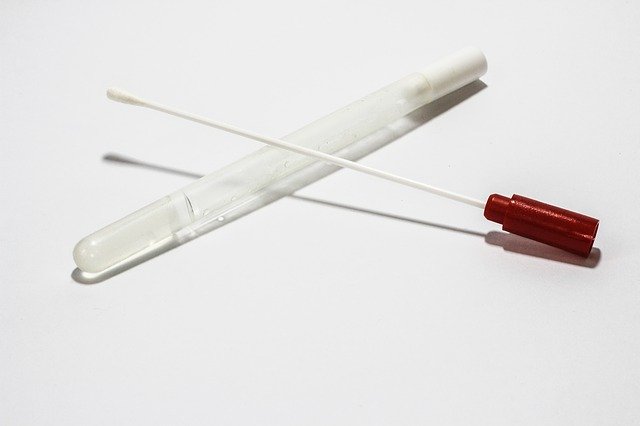
The diagnostic test uses non-invasive, easy to administer, saliva swabs to determine whether or not the ribonucleic acid (RNA) of SARS-CoV-2 virus is present in the sample.
SUNY Upstate president Mantosh Dewan said: “The development of the Clarifi COVID-19 test is also a great example of Quadrant’s ability to nimbly apply its growing expertise in RNA analysis to an urgent healthcare need and expeditiously muster the resources to develop a solution.
“The coronavirus has had a devastating effect on health and welfare worldwide, so it is extremely gratifying for us to take the expertise we have gained working on RNA diagnostic tests for other health conditions, such as autism spectrum disorder and Parkinson’s disease, and apply that expertise to the development of this critically important test.”
Clarifi Covid-19 test kit limits false-negative results using an RNA stabilising solution
Clarifi Covid-19 test kit comprises a saliva collection swab and required reagents to run the analysis.
The company said that its Clarifi Covid-19 Test is a patient friendly, clinically useful and effective for testing laboratories, and is designed to prevent false-negative results through an RNA stabilising solution integrated into the saliva collection kit.
The test is expected to be immediately available at high-complexity clinical laboratories to serve patients through physicians’ offices, urgent care clinics and hospitals.
Quadrant Biosciences founder and CEO Richard Uhlig said: “This stabilizing solution was designed to prevent RNA degradation prior to laboratory analysis – this is critical as Covid-19 is a single-stranded RNA virus and susceptible to degradation by enzymes in mucus and saliva.”
“SUNY Upstate’s work with Quadrant Biosciences, a Start-Up New York company, has led to important breakthroughs in the development of saliva-based diagnostic solutions for neurological conditions such as autism spectrum disorder, Parkinson’s disease and concussion injuries.
“The ability to transfer this innovative approach to address the exigent need for Covid-19 testing solutions, further demonstrates the critical importance of these types of collaborations.”
In August, Fluidigm has received the EUA for its Advanta Dx SARS-CoV-2 RT-PCR Assay to detect SARS‑CoV‑2 virus nucleic acid.
The assay has been developed to test the saliva specimens collected in a sterile container from individuals suspected of Covid-19 infection.






