Monaghan Medical (MMC) has secured approval from the US Food and Drug Administration (FDA) for a new respiratory treatment option.
The regulator has approved a new combination, including Aerobika oscillatory positive expiratory pressure (OPEP) device and VersaPAP positive airway pressure device, to create an effective tandem therapy for the treatment of different pulmonary conditions.
The tandem therapy helps clinicians to treat a range of pulmonary conditions
According to the company, the combination of Aerobika OPEP and VersaPAP devices facilitates better synergy when used in tandem and enhance the function of each.
Aerobika OPEP, VersaPAP device and AeroEclipse II breath-actuated nebulizer (BAN) can be used individually or in different combinations to better treat a patient’s pulmonary condition and adjust therapeutic regimens based on a patient’s condition changes.
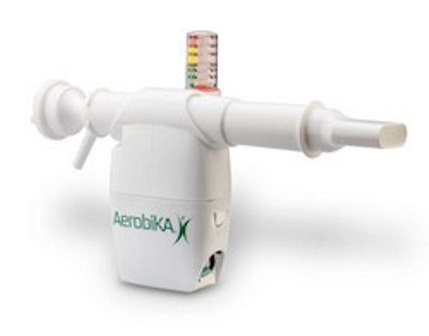
Aerobika OPEP is hand-held and drug-free oscillating positive expiratory pressure device developed to move fluid out of the lungs and extend the airways, as well as improve the delivery of medication by aerosol spray.
After a patient exhales through the device, intermittent resistance will lead to a pressure-oscillation dynamic to expand the airways and mobilise secretions.
Aerobika OPEP device, which will function irrespective of flow rate or the angle in which it is held, will reduce drug loss by facilitating direct aerosol pathway when used in combination with nebulizer therapy. It can be used up to one year.
VersaPAP is a hand-held device that augments supplied air by four times to form positive pressure on inhalation, enabling to draw larger breath.
The additional air creates a slight resistance on exhalation, helping the patient to expand airways and improve gas exchange in the lungs. It also helps in the mobilisation of secretions.
VersaPAP includes an integrated manometer, which offers immediate feedback to both the patient and the clinician, assisting in monitoring and achieving desired airway pressures.
Monaghan Medical is engaged in the development of cost-efficient and outcome-based solutions for its customers.
In June 2019, TransMedics secured an FDA PMA approval for expanded clinical indications of the Organ Care System (OCS) Lung.






