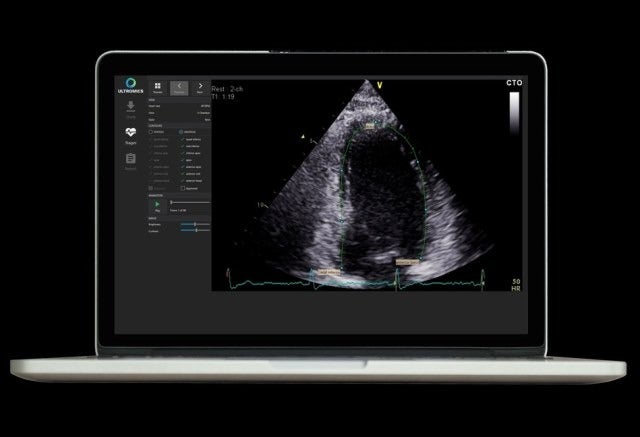
UK-based health technology firm Ultromics has secured 510(k) clearance from the US Food and Drug Administration (FDA) for its EchoGo Core image analysis system.
EchoGo Core is an artificial intelligence (AI) powered decision support system designed to automate the analysis and quantification of ultrasound-based heart scans.
The vendor-neutral platform can be integrated into the medical imaging environment to provide clinicians with results as part of their routine diagnostic workflow.
EchoGo Core will help clinicians to accurately interpret echocardiograms
EchoGo enables clinicians to efficiently and precisely interpret echocardiograms by automating the process and applying its AI analysis for better results at the scans.
The AI will be used by the EchoGo to calculate the left ventricular ejection fraction (EF) and left ventricular volumes (LV), as well as the automated cardiac strain.
Ultromics has partnerships with major cardiology clinical centres in the US and 30 NHS centres in the UK. Through the partnerships, the company is continuously improving its scanning algorithms by analysing the archive of scans held by these centres
Since many years, EchoGo has been under the development, from the first trial being launched in 2011 to Ultromics spinning out of the University of Oxford in 2017.
The company will now focus on the commercialisation of EchoGo Core system.
Ultromics founder and CEO Ross Upton said: “This is an incredibly exciting step towards the future of healthcare, EchoGo will help clinicians make more accurate and informed decisions to improve patient care delivery. It’s truly a watershed moment for Ultromics.
“Strain has shown to be very valuable in cardiovascular diagnostics and has been demonstrated in published studies to be linked with earlier detection of disease and improved patient outcomes.
“Ultromics’ will be the first to use artificial intelligence for automated strain analysis which is applicable to 60 million scans per year. Crucially, strain is also becoming reimbursable from January 2020 in the US.”
In May 2018, Ultromics secured £10m funding to advance the commercialisation of AI technology for the diagnosis of coronary heart disease.






